Research identifies novel mechanism that controls dendritic branch formation in neurons
On May 8, Dr. ZOU Wei’s group published a research article entitled “A Dendritic Guidance Receptor Complex Brings Together Distinct Actin Regulators to Drive Efficient F-Actin Assembly and Branching” in Developmental Cell. In this article, the authors reported an exciting discovery of a new mechanism by which neurons grow high-order dendrite branches, a process essential for the development of neural circuits throughout animals.
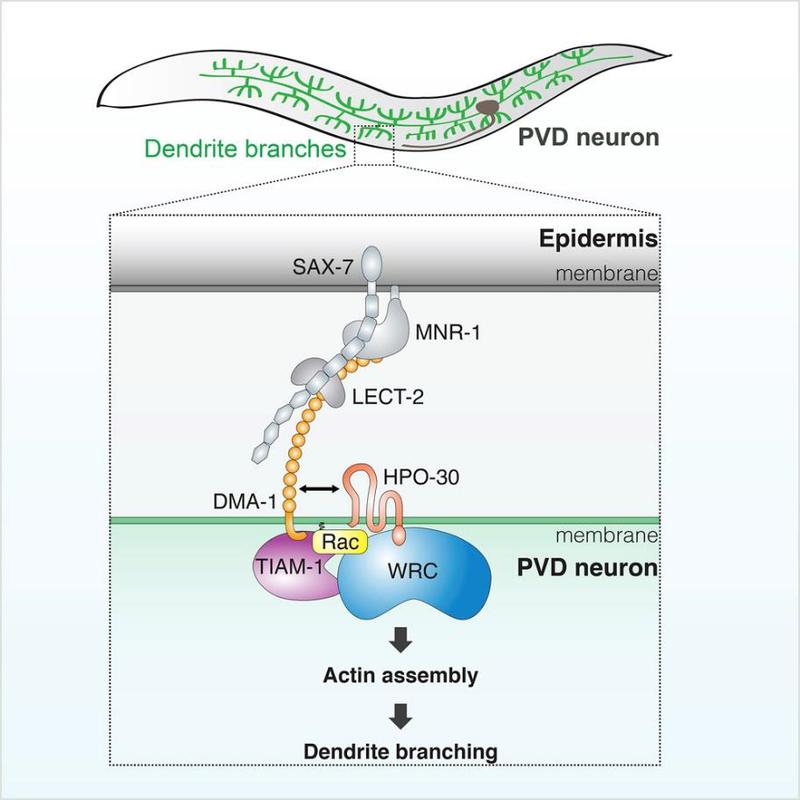
The complicated wiring of numerous neural circuits in our nervous system relies on the ability of neurons to develop highly branched dendritic structures. Formation of branched dendrites is driven by coordinated actions of various extrinsic and intrinsic mechanisms, including transcriptional regulation, intracellular vesicle trafficking, cytoskeleton remodeling and ligand-receptor recognition. However, very little is known about how neurons transmit extrinsic, ligand-receptor signaling to intracellular machineries to direct reorganization of the actin cytoskeleton and drive dendrite formation.
By combining multiple approaches of genetics, cytobiology and biochemistry, the authors revealed how the receptor-ligand complex formed by DMA-1 controls actin cytoskeletal assembly to promote dendrite branching. They found that DMA-1 actually cooperates with a claudin-like membrane protein named HPO-30. The two membrane receptors form a complex at the dendrite branching sites. Each receptor can use its cytoplasmic domain to recruit a distinct actin regulator through direct interactions. DMA-1 recruits an actin regulator named TIAM-1, a Rac GEF that can stimulate actin assembly by activating the Rac GTPase. And HPO-30 recruits another actin regulator, named the WAVE Regulatory Complex (WRC), which can be activated by the Rac GTPase to initiate actin polymerization.
Disrupting the interaction between either DMA-1 and TIAM-1 or HPO-30 and the WRC only partially affected the formation of dendritic branches, whereas disrupting both interactions at the same time completely eliminated high-order branches. Therefore, the receptor complex formed by DMA-1 and HPO30 can bring together two distinct actin regulators, TIAM-1 and the WRC, to close vicinity at membranes to synergistically activate actin assembly, which, in turn, drives dendrite branching.
In summary, the study established a new mechanistic link between membrane receptors and intrinsic signaling pathways that control actin assembly and dendrite branching during neural development. Similar mechanisms are likely used in many other processes for neurons to achieve spatial and temporal control of actin assembly during morphogenesis.
Dr. ZOU Wei (Institute of Translational Medicine, Zhejiang University) and Dr. DONG Xintong (Stanford University) are the co-first authors of the paper. Dr. ZOU Wei, Dr. CHEN Baoyu (Iowa State University) and Dr. Kang Shen (Stanford University& Howard Hughes Medical Institute) are the co-corresponding authors. This project was partially supported by grants of the National Natural Science Foundation of China.
Source:School of Medicine