ZJU professor's depression research published in Nature

According to the World Health Organization, depression is a common illness worldwide, with more than 300 million people affected. It can cause the affected person to suffer greatly and function poorly at work, at school and in the family. At its worst, depression can lead to suicide.
Professor of Neurobiology HU Hailan has been doing impactful research into how emotional and social behaviors are encoded and regulated in the brain. On February 15, her team made a new breakthrough with two articles published on Nature under the titles “Ketamine blocks bursting in the lateral habenula to rapidly relieve depression” and “Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression”.
In the two research articles, Professor HU’s team reported a series of important discoveries, including the rapid antidepressant mechanism of ketamine, the abnormal neural coding pattern and neuron-glia interaction underlying depression.
Ketamine: effective but enigmatic
Classical antidepressants have suffered from the problems of slow action (>6~8 weeks), low efficacy (<30% emission rate) and side effects. The party drug ketamine has recently emerged as a “miracle drug” due to its rapid antidepressant actions and high efficacy on treatment-resistant depression patients.
However, the mechanisms of how ketamine acts so quickly and the exact target of ketamine – which brain region and which cell groups – have remained elusive. Due to its addictive nature and potential side effects, ketamine has not been widely used in clinics. Therefore, elucidating the underlying mechanism of ketamine is essential for developing new rapid-acting antidepressants without side effects.
Read more: Ketamine gaining popularity as a treatment for the severely depressed
Discovering Ketamine’s antagonist
In the first article, HU’s team found that ketamine blocks bursting activity in the lateral habenula (LHb), an anti-reward center in the brain, to rapidly relieve depression. They discovered that in various animal models of depression, the intrinsic burst firing activity of LHb neurons is significantly increased. Burst firing, a particular spike pattern with clusters of high-frequency spikes, provides a robust form of information coding and a stronger output from LHb to inhibit the reward-related dopaminergic and serotonergic centers.
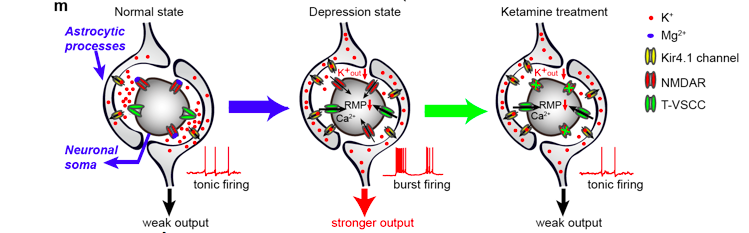
A model for mechanisms of depression and ketamine treatment in LHb: Upregulation of Kir4.1 on astrocytic processes surrounding neuronal soma leads to enhanced K+ buffering at the tight neuron-glia junction, decreased Kout and hyperpolarized neuronal RMP. Consequently, de-inactivation of T-VSCCs initiates NMDAR-dependent bursts, causing a stronger output of LHb to trigger depression. Ketamine blockade of NMDARs stops bursts and relieves depression.
The team discovered that LHb burst firing strongly depends on NMDAR, a major subtype of excitatory glutamate receptors within the brain. Ketamine, as a NMDAR antagonist, completely eliminates LHb bursting in vitro and reduces bursting of depressive-like animals to the level of naïve control animals in vivo.
Collectively, these data suggest a simple and novel mechanism whereby ketamine quickly elevates mood by instantaneously blocking NMDAR-dependent bursting activity of LHb to disinhibit downstream dopaminergic and serotonergic neurons.
Going deeper: molecular determinant of depression
In the second research article, the team further identified the molecular mechanism by which neuron-glia interaction at the LHb regulates burst firing in depression. Through an unbiased, mass spectrometry-based, quantitative proteomic screening, they identified an inward-rectifying potassium channel, Kir4.1, which is upregulated in depression. Kir4.1 is exclusively expressed in the astrocytes, one type of glia cells, in the LHb.
The results point to the important role of a glia-specific ion channel in the regulation of neuronal firing pattern, and provide compelling evidence for Kir4.1 being a powerful molecular determinant of depression.
Far-reaching implications
The two articles, with one addressing how LHb neurons increase burst firing in depression and the other revealing this burst firing to be a prominent target of rapid antidepressant ketamine, make a strong case that the firing mode of LHb neurons is critical in depression.
The newly discovered structure-function mechanism at the glial-neural interface in burst regulation may enrich our understanding of the basic cell biology of how glia regulates neural activity in physiological and pathological states.
Together, they provide a new framework for understanding the molecular, cellular and circuit mechanism of depression and shed important light on developing new rapid-acting antidepressants.
A vibrant team

Authors of the papers: SANG Kangning, YANG Yan, HU Hailan, DONG Yiyan, CUI Yihui, NI Zheyi.
Fascinated by the link between brain and behavior, HU tends to summarize her research into two categories: “the interesting” and “the useful”. For instance, she considered her “Winner Effect” findings as a typical example of “the interesting” while the research on depression falls into the second category.
Notably, it is quite rare for Chinese researchers to have two research articles published side-by-side in Nature. Leading a young but highly prolific team, Professor HU said their secret is good communication and understanding.
“We meet frequently to brainstorm. Apart from working hard in the lab, communicating within the team and with international researchers is equally important,” HU said.
She added: “I would like to thank the School of Medicine and Zhejiang University for enabling us to thrive in a free environment.”
“The team is like a family,” said Professor LI Xiaoming, executive dean of ZJU’s School of Medicine.
People

Professor, Interdisciplinary Institute of Neuroscience and Technology
School of Medicine