A novel approach to "camouflage" for anti-cancer drugs
Antineoplastic drugs work by targeting and killing rapidly dividing cancerous cells, but this process is fraught with several obstacles, such as poor water solubility, off target effects and elimination by immune system. To facilitate a better circulation in the body, produce a more effective treatment or reduce the harmful effect of antineoplastic drugs on normal tissues, researchers have been constantly endeavoring to use nanomaterials to encapsulate antineoplastic drugs in the biochemical field.
Recently, Prof. HUANG Feihe, Prof. MAO Zhengwei from Zhejiang University and Dr. YU Guocan from the American National Institutes of Health developed a novel approach to fabricating a supramolecular peptide that shows programmable self-assembly with multiple morphologies and extensive applications in photodynamic therapy. This new drug delivery system is an ideal vehicle to encapsulate a photosensitizer for photodynamic therapy. Relevant findings are published in the June 3 issue of Nature Communications.
Marked by its high biocompatibility, cell permeability, and low immunogenicity, the peptide is an optimum candidate for functional materials at the nano- or macroscale in the biochemical field. Depending on peptide sequences, physical conditions and external stimuli, peptide amphiphiles are able to self-assemble into fibers, nanotubes, vesicles, and nanoparticles (NPs), and such assemblies have been applied in drug delivery, cell imaging, tissue engineering, and membrane protein stabilization.
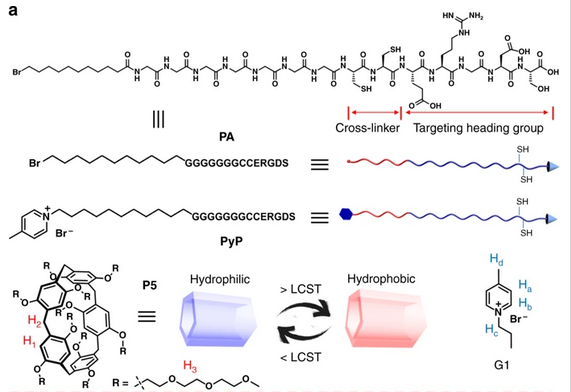
Chemical structures and cartoon representations of PA, PyP, and P5.
Pillararene-based supra-amphiphiles, which bridge the gap between controllable self-assembly and simple synthesis by non-covalent interactions, are being actively investigated. To better control the encapsulation of drugs and the morphology of nanostructures, HUANG Feihe et al. pioneer in introducing pillar[5]arene bearing ten tri(ethyleneoxide) groups (P5) due to its unique thermo-responsive property and strong binding affinity for cationic guests.
A supramolecular peptide can be constructed via pillar[5]arene-based host−guest recognition, thereby streamlining the peptide modification process and promoting the controllability of the self-assembly behavior. Due to peptide sequences on the exterior surfaces and hydrophobic cores of self-assemblies, the nanoparticles formed from the supramolecular peptide are suitable vehicles to encapsulate a photosensitizer for photodynamic therapy.
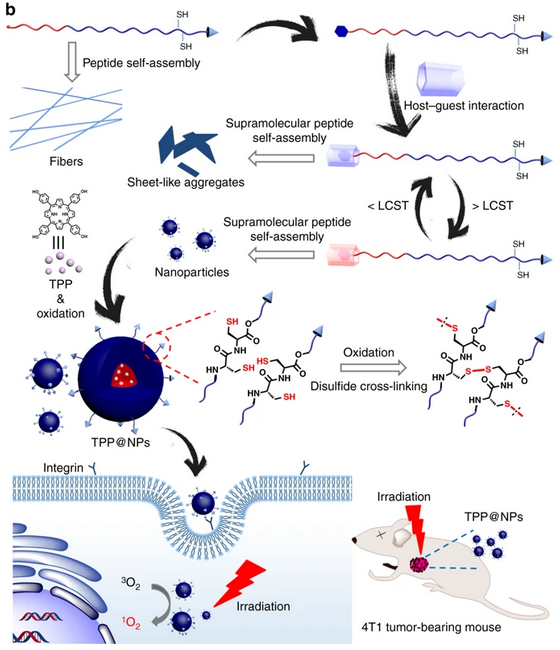
Schematic illustrations of the programmable peptide self-assembly and PDT process. Solid arrows indicate experimental procedure and hollow arrows refer to self-assemblies or structural details.
In vitro and in vivo studies demonstrate that the inherent targeting capability and the supramolecular strategy tremendously enhances the photodynamic therapeutic efficiency of this supramolecular peptide, which thereby holds enormous promise in precise cancer therapy and peptide modification.