Why do well-fed mice keep eating?
A well-fed mouse is dozing off. All of a sudden, a yellow optical fiber in its brain evokes its feeding behavior and it starts to devour food ravenously again.
This scene can be observed in a lab of the School of Medicine, Zhejiang University. Neurologist WANG Hao discovers a set of neurons which can “unplug” mice’s feeding behavior.
The relevant finding is published in the July 16 issue of Cell Reports. This “on/off switch”—GABAergic cells as an inhibitor—is located in the anterior ventrolateral periaqueductal gray (vlPAG). The research article presents how this “switch” regulates feeding in a distinct and precise way.
In the first experiment, mice were observed to keep eating even if they were full. As a general rule, a well-fed mouse tends to have no appetite for food. However, once anterior vlPAG GABAergic cells are selectively inhibited via yellow light, the mouse will forage for food rapidly.
Yellow-light-induced selective inhibition of anterior vlPAG GABAergic cells transfected with halorhodopsin evokes a rapid and obvious feeding behavior in well-fed mice. A continuous 590 nm laser is given as indicated by the appearance of the yellow “laser on” text.
In the second experiment, 24 h food-deprived mice were observed to undergo a cessation of ongoing feeding. Normally, a starving mouse is itching for food. However, when GABAergic cells were activated, the situation was reversed. A voracious mouse remained indifferent to food and even put down its food without the slightest hesitation in the context of photo-activation.
Selective photo-activation of the anterior vlPAG GABAergic cells produces an immediate cessation of ongoing feeding in 24 h food-deprived mice. The duration of pulsed 470 nm laser is indicated by the appearance of the blue “laser on” text.
“This tells us that these GABAergic cells play a crucial role in mediating food intake,” WANG Hao said, “Food intake is one of the critical issues for scientists. With the development of society, food rich in calories is increasingly cheap, which contributes to the prevalence of obesity. Scientists are thus striving to explore how the brain controls feeding behavior so as to tackle obesity, anorexia and other associated health problems.
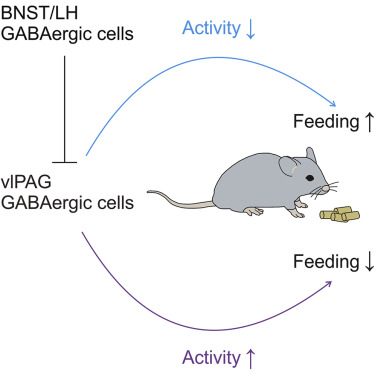
Nonetheless, eating is extremely complicated. Previous studies have shown that multiple circuits extensively interact with each other in the regulation of the appetite. Among such circuits, the Agouti-related protein (AGRP)-expressing neurons in the hypothalamic arcuate nucleus (ARC) and the lateral hypothalamus (LH) have been suggested to be the most prominent. However, the downstream pathways of the hypothalamus and the non-canonical neural circuitry involved in feeding behavior remain largely uncharacterized. “Hunger and gluttony are two different motives, with the former seeking for energy and the latter happiness,” WANG Hao said.
WANG Hao et al. discover that suppressing the activity of GABAergic cells in vlPAG, whether directly or through long-projection GABAergic inputs from either the bed nucleus of the stria terminalis (BNST) or LH, sufifices to promptly induce feeding behavior in well-fed mice. In contrast, optogenetic activation of these cells interrupts food intake in starving mice. Long-term chemogenetic manipulation of vlPAG GABAergic cell activity elicits a corresponding change in mouse body weight.
This study reveals distinct midbrain GABAergic pathways and highlights a vital role of GABAergic cells in the anterior vlPAG in feeding behavior. It may well provide insights into developing drugs for obesity.