Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy, scientists find
Anticancer nanomedicines, such as liposomal doxorubicin, are supposed to be delivered to the cytosol of cancer cells in solid tumours like a “package”. However, at present, clinically-used nanomedicines can do nothing more than reduce their side effects, thus unable to improve their therapeutic efficacy significantly. One of the major reasons is that nanomedicines fail to penetrate into every cancer cell in tumors in a “door-to-door” manner.
Then, how to deliver nanomedicines to cancer cells directly? Recently, the team led by Prof. SHEN Youqing with the College of Chemical and Biological Engineering at ZJU and the team led by GU Zhen with California NanoSystems Institute and Center for Minimally Invasive Therapeutics at UCLA engaged in collaborative research into this field. They put forward a new mechanism for the active penetration of nanomedicines into tumour cells. To address this challenge, they needed to ensure that nanomedicines in solid tumours can be delivered into every cancer cell through different animal models. Their findings are published in the July 01 issue of Nature Nanotechnology.
Typically, nanomedicines range in size from 10 to 100 nm, resulting in a more restrictive molecular diffusion from the perivascular regions to distal cells. Tuning the size of nanomedicines and other properties could somewhat improve tumour penetration; however, the efficiency remains constantly low due to the unchanged passive diffusion of bulky nanomedicines against the interstitial fluid pressure gradient through a packed paracellular matrix.
Inspired by the endothelial cell caveolae-mediated transcytosis that actively transports cargos across the endothelium, SHEN Youqing et al. proposed that the endothelial cell transcytosis may actively transport nanomedicines across the capillary wall into tumour tissues, in addition to the passive accumulation via the enhanced permeation and retentioneffect. Furthermore, this ATP-dependent transcytosis process could also circumvent the difficulty in passive diffusion and make nanomedicines actively infiltrate throughout the tumour to reach distal cells.
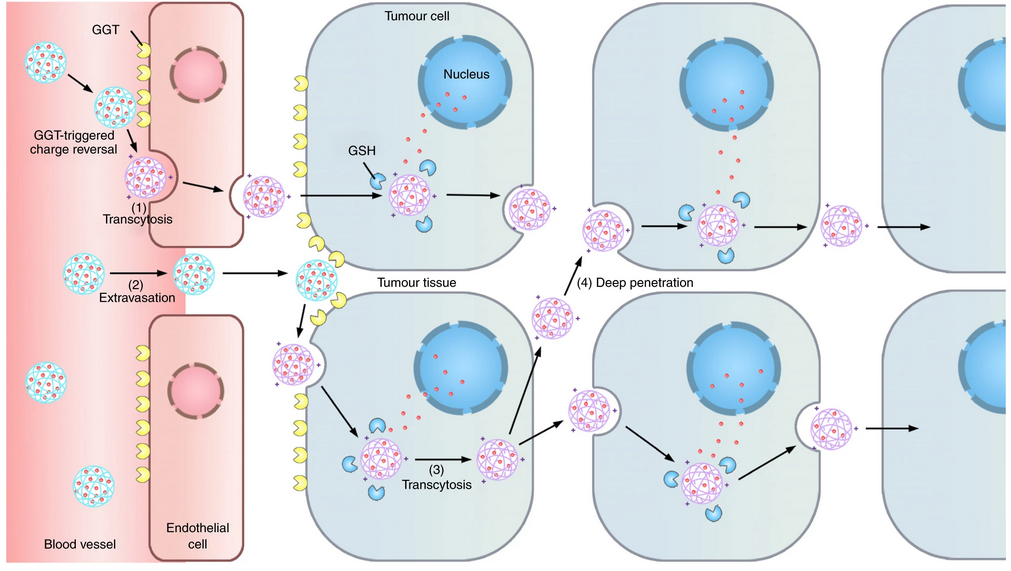
The neutral long-circulating nanomedicine is converted into a cationic one by the enzyme on the luminal endothelial cell surface, which triggers the AMT of the nanomedicine across the endothelium.
SHEN Youqing et al. presented a γ-glutamyl transpeptidase (GGT)-responsive camptothecin— polymer conjugate that can actively infiltrate throughout the tumour tissue through transcytosis. When the conjugate passes on the luminal endothelial cells of the tumour blood vessels or extravasates into the tumour interstitium, the overexpressed GGT on the cell membrane cleaves the γ-glutamyl moieties of the conjugate to generate positively charged primary amines. The resulting cationic conjugate undergoes caveolae-mediated endocytosis and transcytosis, which enables transendothelial and transcellular transport and a relatively uniform distribution throughout the tumour. The conjugate shows a potential antitumour activity in mouse models that lead to the eradication of small solid tumours (~100 mm3) and regression of large established tumours with clinically relevant sizes (~500 mm3), and significantly extends the survival of orthotopic pancreatic tumour-bearing mice compared to that with the first-line chemotherapeutic drug gemcitabine.
This bioresponsive drug delivery strategy can successfully circumvent a series of biological barriers in a ‘CAPIR’ (circulation, accumulation, penetration, internalization and release) cascade and conquer the constraints of nanomedicines in diffusion, thereby opening up a new avenue for the designing of nanomedicines in the next phase.