“Seeing through” the structure of human KCC opens the door to epilepsy treatment
The homeostasis of potassium, sodium and chlorine in human cells is subjected to rigorous regulation. Once the homeostasis is knocked off balance, it will trigger a series of diseases such as hypertension, depression and epilepsy. On the cell membrane, there is a kind of protein called cation-chloride cotransporter (CCC), which can take ions into and out of cells, thus effectively regulating the ion homeostasis in cells. However, for lack of precise information about its structure, people have long been unable to know much about the working mechanism of this kind of protein.
Recently, the research team led by GUO Jiangtao from the Zhejiang University School of Medicine has successfully deciphered the cryo–electron microscopy (cryo-EM) structure of human potassium-chloride cotransporter KCC1 in potassium chloride or sodium chloride at 2.9- to 3.5-angstrom resolution. Their research reveals one potassium site and two chloride sites in KCC1, enabling them to create a feasible model for a potential ion transport mechanism in KCCs and providing a blueprint for drug design.
Relevant research findings are published in the October 25 issue of the journal Science. Lead co-authors include Dr. LIU Si from the School of Medicine, Dr. CHANG Shenghai from the Center of Cryo-Electron Microscopy and HAN Binming, a master’s student in the Department of Physics.
As a general rule, the concentration of intracellular K+ is higher than that of extracellular ones. KCCs use this concentration gradient of K+ to transport intracellular K+and Cl- to extracellular domains, thus regulating the concentration of intracellular Cl-.
The concentration of Cl- is an exceedingly crucial indicator. For example, in the process of γ-aminobutyric acid (GABA)–mediated modulation, neurons need to maintain a relatively low Cl−concentration so as to fulfill their functions as inhibitors. It is KCC2, one of the K-Cl cotransporters, that participates in cell volume regulation by transporting ions across the plasma membrane to reduce the osmotic difference when cells are exposed to hypertonic or hypotonic environments. Considering their important functions in ion transport, it is not surprising that mutations in KCCs lead to a wide spectrum of human diseases. For example, five single amino acid substitutions in KCC2 can cause human epilepsy.
Despite the fact that KCCs play such a vital role, their structure has long remained elusive for two major reasons: the inaccessibility of KCC samples and the difficulty in analyzing a high-resolution structure. With persistent and determined efforts, GUO Jiangtao et al. have successfully dissected the structure of human KCC1.
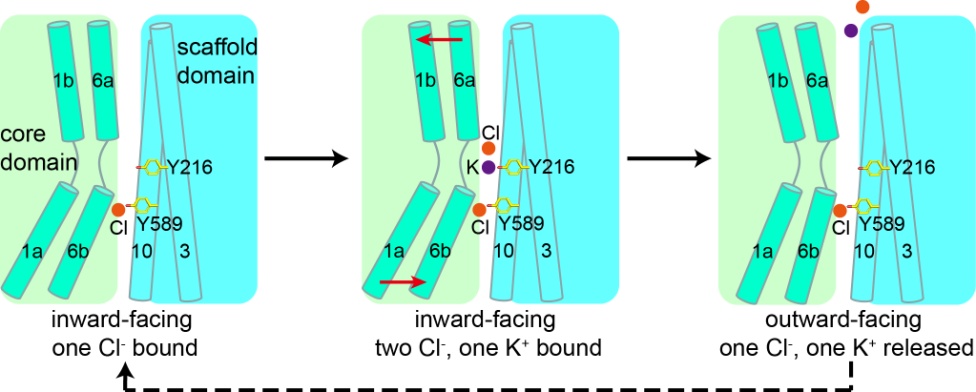
They present the cryo-EM structures of KCC1 in potassium chloride or sodium chloride at 2.9- to 3.5-angstrom resolution. KCC1 exists as a dimer, with both extracellular and transmembrane domains involved in dimerization. The structural and functional analyses, along with computational studies, reveal one potassium site and two chloride sites in KCC1 which are essential for the ion transport activity. KCC1 adopts an inward-facing conformation, with the extracellular gate occluded.
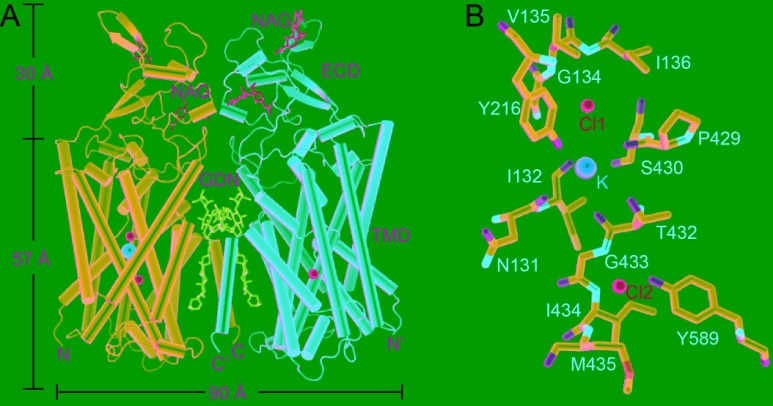
The structural and functional characterization of KCC1 contributes to the development of a K-Cl cotransport model. The KCC1 structure provides a framework to interpret disease-related mutations of other KCCs such as KCC2 and KCC3, whose mutations cause genetic disorders of the human nervous system. It is expected that the structural studies of human KCC1 will promote drug development and disease treatment targeting human CCCs.