Peripheral immune cells under stress cause anxiety-like behavior, a study shows
The central nervous system was previsouly assumed as the major “culprit” behind depression and anxiety. Little attention was paid to the role that other organs played in this process. Recently, researchers from the JIN Jin Lab at the Zhejiang University Life Sciences Institute found that physical stress-induced leukotriene B4 triggers severe mitochondrial fission in CD4+ T cells, which further leads to a variety of behavioral abnormalities including anxiety, depression, and social disorders. Their research findings are published in the October 31 issue of the journal of Cell.
Co-lead authors are PhD student FAN Keqi and Dr. LI Yiyuan at the Zhejiang University Life Sciences Institute, and co-corresponding authors are Prof. JIN Jin from Zhejiang University and Prof. CHAI Renjie from Southeast University.
In today’s world, science and technology is developing at an expeditious pace and people’s living standards are on the rise. However, people’s inner peace and happiness have not grown accordingly, and virtually everyone feels more or less stressed out. Constant exposure to psychological stress will increase the risk of depression and anxiety. Severe anxiety will even drag people into a vicious circle, generating incessant internal friction at spiritual and physical levels and eventually causing irrevocable damage.
In normal conditions, CD4+ T cells are the guardian of health for organisms. In this study, JIN Jin et al. found that T cells “defected from the battlefield” and caused anxiety. Existing studies reveal that the central nervous system is an immune-privilege organ. Because it is exceedingly vital, the animal body evolves a blood-brain barrier to block peripheral immune cells outside the nerve center and prevent these cells from interfering with the normal work of neurons in order to avoid external influences.
Then how do CD4+ T cells break though the blood brain barrier and govern the nerve center? It turns out that under normal circumstances, mitochondria will propel glucose into releasing energy through glycolysis, converting glucose into pyruvic acid which can thus enter the citric acid cycle to provide energy for normal cells. Researchers found that glucose is not metabolized via a normal glycolytic pathway in CD4+ T cells with mitochondrial fission, but that a large amount of purine (xanthine) is synthesized via the pentose pentaphosphate pathway. Unlike T cells, xanthine can easily pass through the blood-brain barrier to reach the amygdala, the emotional processing center of the brain. Xanthine acts on oligodendrocytes in the amygdala through purine receptors on the cell surface, causing the abnormal activation and proliferation of oligodendrocytes and eventually the hyper-activation of local neurons in the fear center and severe anxiety in mice.

How does the immune system interfere with the nervous system? At first, the JIN Jin Lab was not 100 percent sure to answer this question. The state-of-the-art high-throughput sequencing technology rendered it possible to integrate various histological data. Therefore, by virtue of a series of big data tools, researchers devised an immune-brain map, which helped them find the clue to the connection between T cells and the nervous system.
“There are myriad types of brain cells. But for single cell sequencing, it would be hardly possible to figure out whom T cell-derived xanthine acts on and what changes have transpired in the brain,” JIN Jin said, “Cutting-edge tools enable scientists to look at the relationship between physiological changes and cells from a wider perspective.”
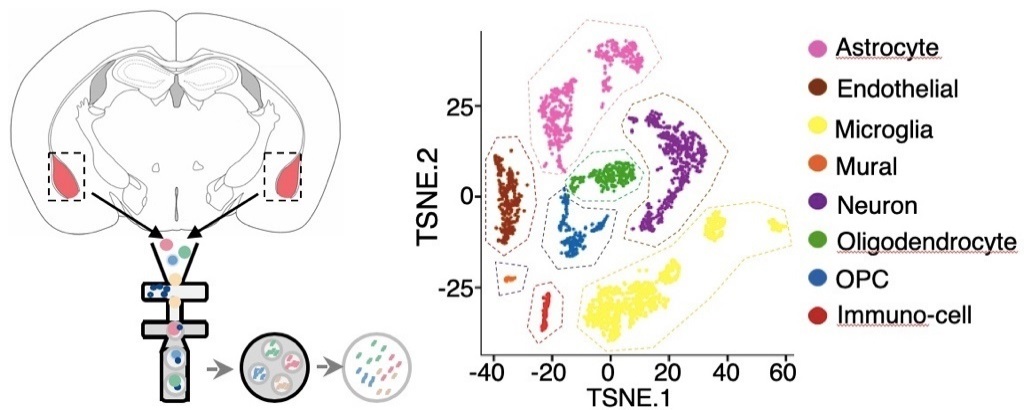
“With the progress of technology, we are able to see the state of each cell and discover new information. In our study, we have also detected an intimate connection between peripheral T cells and oligodendrocytes,” JIN Jin said.
“Only with a clear scientific question in mind and the appropriate use of scientific tools can we do more with less,” JIN Jin added.
This research establishes peripheral CD4+ T cells as pivotal mediators of stress-induced mood disorders. In the future, it will be intriguing to clarify whether a specific CD4+ T cell subpopulation regulates emotions and behavior in anxious patients. It is also essential to get a better picture of the mechanism by which LTB4 promotes the mitochondrial morphology of CD4+ T cells.
These results provide insights into the physiological function of adaptive immunity in neurodevelopment and neuropsychiatric disorders. It is expected that they will create profound implications for developing a valuable therapeutic approach to various psychiatric and metabolic diseases.
