Post-translational modification of PGK1 found as a key signal in tumor growth
By switching the energy source from mitochondrial oxidative phosphorylation to glycolysis, cancer cells gain sufficient biomass-building materials for cell growth and proliferation, and combat highly oxidative microenvironment for better survival. This metabolic reprogramming, also known as the Warburg effect, has shown to contribute to tumorigenesis in a variety of cancers, and become a promising target for diagnosis and targeted therapy of cancer. However, how cancer cells coordinate glucose metabolism through glycolysis and mitochondrial TCA cycle remains largely elusive.
O-GlcNAc is a prevalent post-translational modification (PTM) on serine and threonine residues of proteins. Since O-GlcNAcylation is critical for normal physiology, its aberrant expression is closely associated with a number of diseases, including neurodegenerative diseases, diabetes, cardiovascular diseases, and cancers. However, the mechanism involved in the tumor pathology has yet to be detected at the molecular level.
The research team led by Prof. YI Wen of the Zhejiang University College of Life Sciences published a research article entitled “O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth” in the journal of Nature Communications on January 7.
PGK1, the first ATP-generating enzyme in glycolysis, catalyzes the conversion of 1,3-diphosphoglycerate (1,3-BPG) to 3-phosphoglycerate (3-PG) and produces one molecule of ATP. PGK1 expression is up-regulated in many types of human cancer, but the detailed mechanism by which PGK1 contributes to cancer development is far from being well-understood.
In this study, researchers identified an unknown regulatory mechanism of PGK1 by O-GlcNAcylation in cells. They observed that PGK1 is dynamically O-GlcNAcylated at T255, and this site-specific glycosylation enhances PGK1 activity and simultaneously induces PGK1 translocation into mitochondria. Inside mitochondria, PGK1 acts as a kinase to inhibit pyruvate dehydrogenase (PDH) complex to reduce pyruvate utilization and suppress oxidative phosphorylation. Blocking T255 O-GlcNAcylation on PGK1 suppresses the Warburg effect, decreases colon cancer cell proliferation, and inhibits tumor growth in nude mice. Moreover, O-GlcNAcylation of PGK1 is significantly elevated in human colon cancer tissues compared with the adjacent matching tissues.
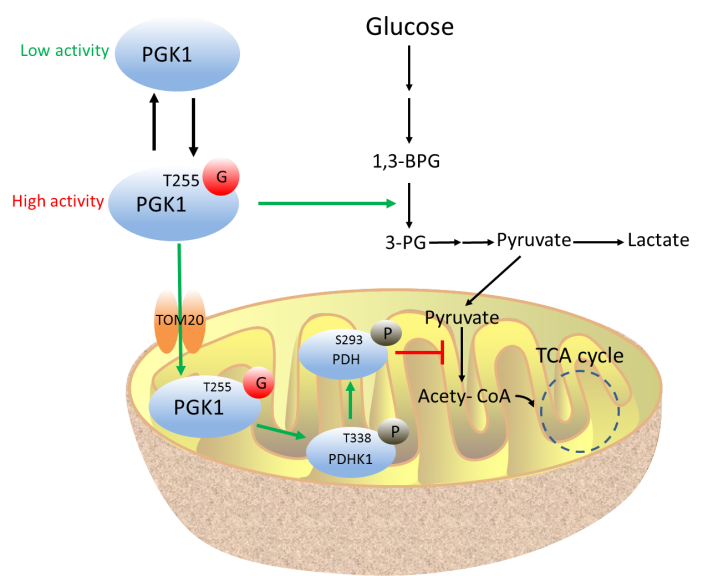
A graphic model of PGK1 O-GlcNAcylation in coordination of glycolysis and TCA cycle to promote tumor growth
This study highlights O-GlcNAcylation as a crucial signal for coordinating glycolysis and the TCA cycle to promote tumorigenesis.