First human trial of COVID-19 vaccine is safe, induces rapid immune responses
On May 22, the journal of The Lancet published relevant data about the trial of the new Ad5 vectored COVID-19 vaccine tested in humans, headed by Prof. CHEN Wei (a distinguished member of the Class of ’98 in Chemical Engineering at Zhejiang University) from the Beijing Institute of Biotechnology and Prof. ZHU Fengcai from Jiangsu Provincial Center for Disease Control and Prevention. The study of 108 adults finds that the first COVID-19 vaccine to reach the phase 1 clinical trial is safe, well-tolerated, and able to generate an immune response against SARS-CoV-2 in humans.
The open-label trial in 108 healthy adults demonstrates promising results after 28 days—the final results will be evaluated in six months. Further trials are needed to tell whether the immune response it elicits will effectively protect against SARS-CoV-2 infection.
“These results represent a significant milestone. The trial demonstrates that a single dose of the new adenovirus type 5 vectored COVID-19 (Ad5-nCoV) vaccine produces virus-specific antibodies and T cells in 14 days, making it a potential candidate for further investigation,” says Prof. CHEN Wei, “However, these results should be interpreted cautiously. The challenges in the development of a COVD-19 vaccine are unprecedented, and the ability to trigger these immune responses does not necessarily indicate that the vaccine will protect humans from COVID-19. This result shows a promising vision for the development of COVID-19 vaccines, but we are still a long way from this vaccine being available to all.”
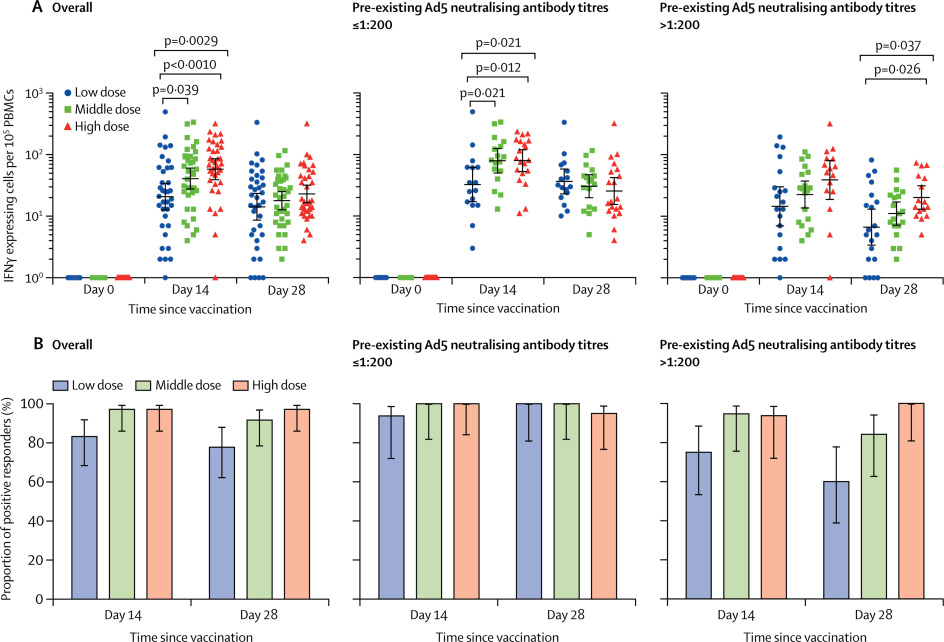
Specific T-cell response measured by ELISpot
The development of an effective vaccine is perceived as a long-term solution to containing the COVID-19 pandemic. Currently, there are more than 100 candidate COVID-19 vaccines in development worldwide.
The new Ad5 vectored COVID-19 vaccine evaluated in this trial is the first to be tested in humans. It uses a weakened common cold virus (adenovirus, which infects human cells readily but is incapable of causing disease) to deliver genetic material that codes for the SARS-CoV-2 spike protein to the cells. These cells then produce the spike protein, and travel to the lymph nodes where the immune system creates antibodies that will recognize that spike protein and fight off the coronavirus.
“Our study has found that pre-existing Ad5 immunity could slow down the rapid immune responses to SARS-CoV-2 and also lower the peaking level of the responses. Moreover, high pre-existing Ad5 immunity may also have a negative impact on the persistence of the vaccine-elicited immune responses,” says Prof. ZHU Fengcai.
Researchers note that the main limitations of the trial are its small sample size, relatively short duration, and lack of randomized control group, which limits the ability to pick up rarer adverse reactions to the vaccine or provide robust evidence for its ability to generate an immune reaction. Further research will be needed before this trial vaccine becomes available to all.
A randomized, double-blinded, placebo-controlled phase 2 trial of the Ad5-nCoV vaccine has been initiated in Wuhan to determine whether the results can be replicated, and if there are any adverse events up to 6 months after vaccination, in 500 healthy adults—250 volunteers given a middle dose, 125 given a low dose, and 125 given a placebo as a control. For the first time, this will include participants over 60 years old, an important target population for the vaccine.