Light activated gene delivery nanodot system to enhance osseointegration
Due to poor osseointegration, patients with osteoporosis or diabetes show high risk of implantation, and often require dental implants with improved osseointegration capabilities. Therefore, there is an urgent need to develop implants that promote osseointegration.
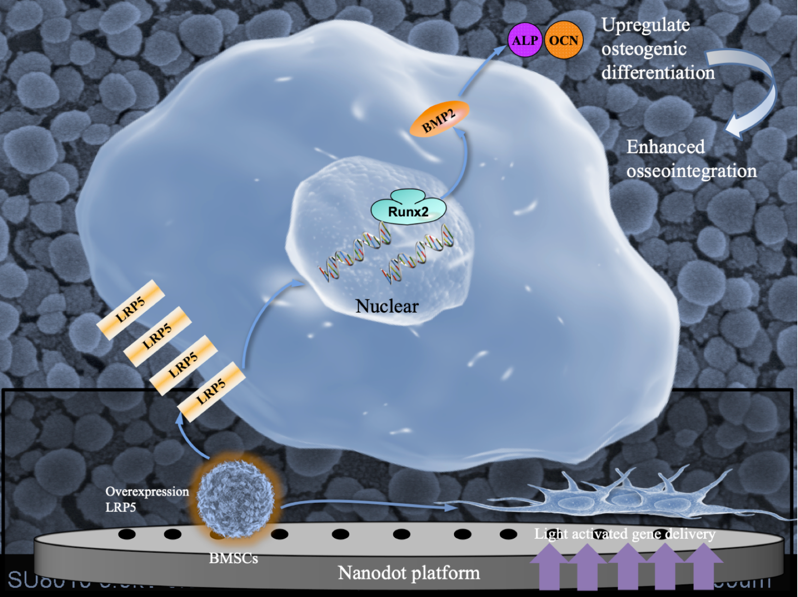
Cell sheets play a vital role in gene therapies. Nonetheless, gene delivery platforms based on cell sheet technology are restricted to small genes. Low-density lipoprotein receptor-related protein 5 (LRP5) is a representative large gene. Due to its large gene sequence, it is rarely used for gene therapy in vivo.
The research team led by Dr. YANG Guoli from the Affiliated Stomatology Hospital of the Zhejiang University School of Medicine developed a light-activated gene delivery platform and evaluated the effects of LRP5-modified bone marrow mesenchymal stem cell (BMSC) sheet coating on osseointegration. Their research findings are published in a research article titled “An effective light activated TiO2 nanodot platform for gene delivery within cell sheets to enhance osseointegration” in Chemical Engineering Journal.
TiO2 nanodot is one of the most extensively applied materials with good photofunctionalization. Anatase TiO2 can promote cell adhesion and osteogenic differentiation of BMSCs. It can be activated through illumination and electron/hole (e-/h+) pairs and reactive oxygen species can lead to the accumulation of electrons. In addition, the structure of absorbed proteins shifts from the α-helix to the β-sheet. These light effects can induce membrane disturbances to improve the gene delivery of adenovirus and pDNA. Light between 300 and 365 nm is prone to be absorbed by TiO2 and can provide energy to overcome the band gap. The 365 nm light does minimal damage to DNA. The TiO2 nanodot platform illuminated by 365 nm light may well become a potential tool for gene delivery.
YANG Guoli et al. developed a nanodot platform using light and adenoviruses to harvest LRP5-overexpressing BMSC sheets with a nearly 2-fold increase in efficiency and a 40% reduction in time compared with a traditional gene delivery platform. Overexpression of LRP5 regulated the expression levels of alkaline phosphatase (ALP), bone morphogenetic protein-2 (BMP2), and runt-related transcription factor-2 (Runx2). Furthermore, in vivo results suggested that LRP5 enhanced BMSC sheet-mediated osseointegration. This light-activated gene delivery nanodot platform holds great promise for introducing various genes and increasing the scope of application for cell sheet-based gene therapies.