Pharmacological targeting of MCL-1 promotes mitophagy to treat Alzheimer’s disease
Alzheimer’s disease (AD) is a severe neurodegenerative disorder that exerts an extremely adverse impact on individuals, families and society at large, with symptoms such as memory loss, cognitive dysfunction mood swings and loss of kinetic abilities. At present, there are about 50 million people worldwide with AD and this number is projected to soar to 152 million. AD is one of the most financially costly diseases. Currently, 1 trillion dollars or so is spent annually worldwide on the treatment and care of Alzheimer’s patient, and this figure is expected to double by 2030.
The causes of AD are very complicated. Popular notion has it that AD occurs when abnormal amounts of proteins, amyloids and possibly tau proteins, form in the brain and begin to encroach upon the organ’s cells. Since 1998, more than 100 drugs have been tested in clinical trials to treat AD, but merely 6 drugs have been approved by the FDA for marketing. Pitifully, from 2012 to 2020, several giant pharmaceutical companies, including Roche, Eli Lilly, Mersal, Johnson & Johnson, etc., announced the failure or termination of drug development for the treatment of AD, which has cast a dense shadow over mankind’s hope of curing AD.
The research team led by Prof.XIA Hongguang from the Zhejiang University School of Basic Medical Sciences / Liangzhu Laboratory has long been committed to research into the role of selective autophagy in the regulation of major diseases and drug development. Previous studies have shown that a large number of damaged mitochondria exist in the brain cells of Alzheimer’s patients and that mitochondrial dysfunction is closely related to the development of AD. On this basis, they proposed a hypothesis that the recovery of mitochondrial dysfunction may help cure AD. Mitophagy is a selective autophagy pathway for mitochondrial quality control, so they focused their attention on inducing mitophagy. Their findings have recently been published in the journal of Nature Communications.
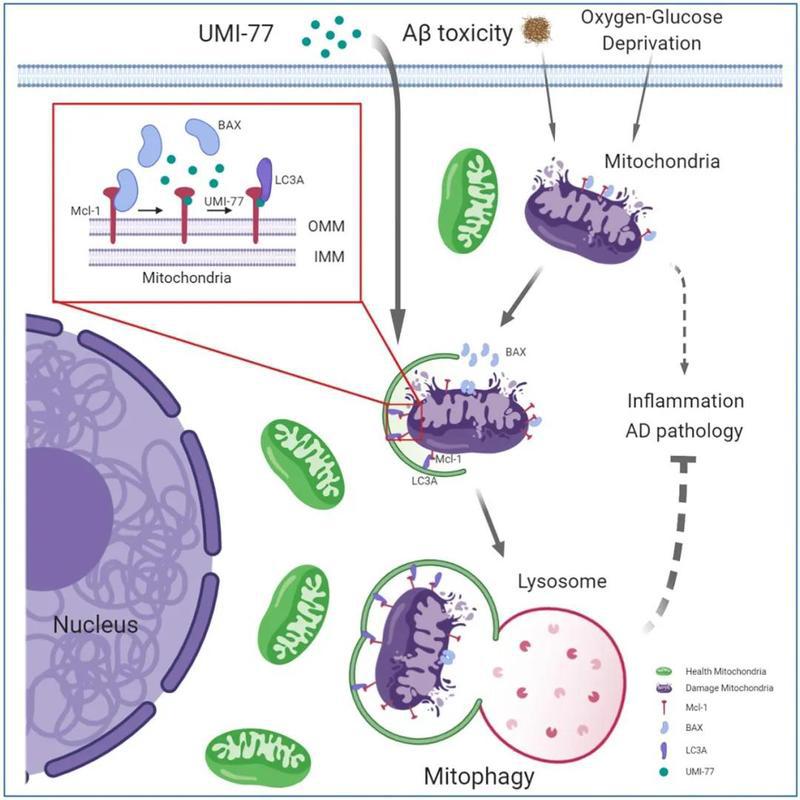
In their research, they first screened a library of 2024 FDA-approved drugs or drug candidates, revealing UMI-77 as a potent mitophagy activator. UMI-77 is an established BH3-mimetic for MCL-1 and is developed to induce apoptosis in cancer cells. They found that at sub-lethal doses, UMI-77 potently induces mitophagy, independent of apoptosis. Their mechanistic studies revealed that MCL-1 is a mitophagy receptor and directly binds to LC3A. Finally, they found that UMI-77 can induce mitophagy in vivo and that it effectively reverses molecular and behavioral phenotypes in the APP/PS1 mouse model of Alzheimer’s disease.
Their findings shed light on the mechanisms of mitophagy, revealing that MCL-1 is a mitophagy receptor that can be targeted to induce mitophagy, and identifying MCL-1 as a drug target for therapeutic intervention in Alzheimer’s disease.