Scientists discover new transmission mechanism of exosome-mediated sorafenib-resistance in hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the most common primary liver tumor with an increasing global incidence. In 2018, HCC was the sixth most frequently diagnosed cancer and the fourth leading cause of cancer-related death worldwide. Sorafenib is the first FDA-approved targeted therapy for advanced HCC. A previous study showed that sorafenib prolonged the median overall survival (OS) by 2.3−3 months in advanced HCC patients that did not qualify for liver transplantation or resection. However, sorafenib resistance in HCC is usually observed within 6 months of treatment, thereby making the follow-up therapy rather formidable.

Recently, the research team led by Prof. CAI Xiujun from Sir Run-Run Shaw Hospital affiliated with the Zhejiang University School of Medicine has made breakthroughs in sorafenib resistance. Their findings are published as a featured cover research article entitled “CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1” in Signal Transduction and Targeted Therapy, elucidating the crucial role of exosome-transmitted circRNA-SORE in sorafenib resistance and its functioning mechanism.
The research team established sorafenib-resistant HCC cell lines, confirmed sorafenib resistance in these cell lines in a real-time manner, and identified deregulated circRNAs through a CircRNA Array analysis. As a result, they found that circRNA_104797 had the most impact on sorafenib resistance in HCC cells so that renamed it as circRNA-SORE (a circular RNA upregulated in sorafenib-resistant HCC cells). They discovered that knocking down of circRNA-SORE led to remarkable cell growth inhibitory effects in sorafenib-resistant HCC cells but had less of an impact on parental cells, further confirming a specific role of circRNA-SORE in the maintenance of sorafenib resistance.
To further investigate potential interacting protein(s) of circRNA-SORE involved in sorafenib resistance, the team carried out mass spectrum analysis on circRNA-SORE pull-down samples and identified 179 circRNA-SORE-binding proteins. To identify proteins regulated by circRNA-SORE, they performed mass spectrum analysis on lysates from circRNA-SORE-silenced cells and identified 129 upregulated and 301 downregulated proteins. Among them, they found that YBX1, as a binding protein for circRNA-SORE, diminished significantly after silencing of circRNA-SORE. To better clarify the binding site of circRNA-SORE and YBX1, they performed a series of immunoprecipitation experiments, including mutant expression, transfection of morpholino antisense oligos (MAO) and confocal imaging. In vitro cellular assays and clinical data analysis revealed that circRNA-SORE promotes drug resistance by increasing YBX1, while knockdown of circRNA-SORE significantly increases YBX1 degradation.
There are various pathways of protein degradation in the human body, such as the lysosomal pathway, the ubiquitin-proteasome pathway and the caspase degradation pathway. Then how on earth can a circular RNA increase the degradation of YBX1? This question had baffled the research team for a long time until they observed a subtle difference in the YBX1 binding-protein profile after knocking down of circRNA-SORE and verified it in meticulously designed experiments. Upon knockdown of circRNA-SORE, the interactions between Pre-mRNA Processing Factor (PRP19) and YBX1 increased. The PRP19 complex, a ubiquitin ligase, mediates protein degradation through the ubiquitin-proteasome pathway. Experiments suggested that PRP19-mediated YBX1 ubiquitination and degradation is involved in the circRNA-SORE-YBX1 regulation. In detail, the team found that circRNA-SORE binds to YBX1 in the cytoplasm, thus preventing YBX1 from translocating to the nucleus to interact with and subsequently be degraded by PRP19. Of note, it is the first report demonstrating PRP19-mediated YBX1 ubiquitination and degradation.
Recent studies demonstrated that tumor-derived exosomes could function as transmitters of drug resistance in different malignancies. Moreover, exon-derived circRNAs were found to be stably enriched in exosomes. Researchers found that adipose exosomes carrying circRNAs could promote the growth of HCC by targeting USP7. “In line with these findings, we found that circRNA-SORE is enriched and transmitted by exosomes, enabling the spread of sorafenib resistance among HCC cells,” said CAI Xiujun. “The stable properties of exosomes and circRNAs may make exosome-carrying-circRNA-SORE a promising biomarker in liquid biopsy for the early detection and noninvasive surveillance of HCC sorafenib resistance.”
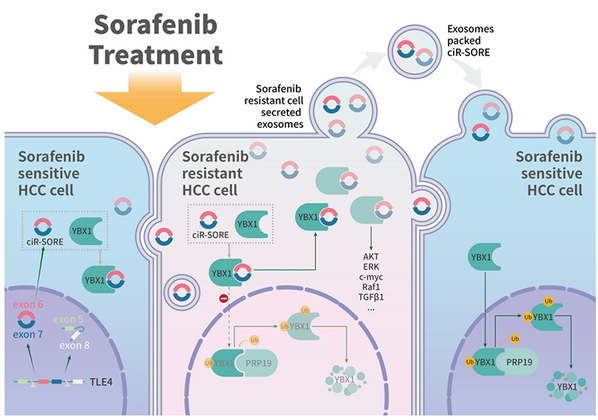
Together, these findings demonstrated that circRNA-SORE plays a critical role in the maintenance and transmission of sorafenib resistance in HCC, that circRNA-SORE functions by preventing PRP19-mediated YBX1 degradation, thereby affecting the expression of downstream gene targets of YBX1, and that circRNA-SORE is transported by exosomes, which enables transmission of sorafenib resistance among HCC cells. Research also revealed that local siRNA-mediated silencing of circRNA-SORE effectively overcomes sorafenib resistance by increasing therapeutic efficacy in different HCC mouse models. This study thus provides a proof-of-concept demonstration for a potential strategy to overcome sorafenib resistance in HCC patients by targeting circRNA-SORE or YBX1.