Scientists create single-cell deep-immune atlas of COVID-19 and discover its immunopathology
As COVID-19 has been raging across the globe in the past year, it has not only constituted tremendous hazards to the safety and well-being of human beings but also incurred immeasurable economic losses. Although most of COVID-19 patients remain asymptomatic or experience only mild symptoms, about 20% suffer overt pneumonia and roughly 5% cases progress to life-threatening Acute Respiratory Distress Syndrome (ARDS) and severe or atypical systemic inflammation. Then how does COVID-19 differ from common pneumonia in pathology and what factors may contribute to the progression into severe symptoms?
Recently, QIAN Junbin, a researcher from Women’s Hospital affiliated with the Zhejiang University School of Medicine, teamed up with Prof. Diether Lambrechts from the VIB Center for Cancer Biology and KU Leuven in Belgium to engage in research into the distinction between mild and critical COVID-19. Their research findings were published in the journal Cell Research.
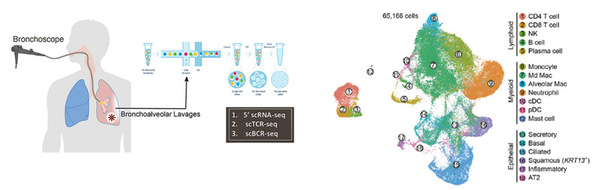
In this study, researchers performed scRNA-seq on bronchoalveolar lavage (BAL) from 22 patients with a positive qRT-PCR for SARS-CoV-2 on a nasopharyngeal swab or a lower respiratory tract sample. They also collected BAL from 13 patients with clinical suspicion of COVID-19 pneumonia, yet negative PCR on lower respiratory tract sampling for SARS-CoV-2. Using scRNA-seq data derived from BAL, they performed deep-immune profiling of the adaptive and innate immune cell landscape within the main locale of COVID-19 pathology. It is the first time that researchers have made a systematic comparison between mild and critical disease groups in lung-localized pathology and revealed the dynamic immunological mechanism for the progression from mild to severe COVID-19 at single-cell resolution.
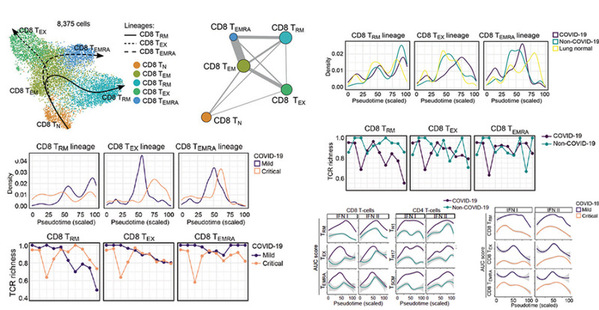
Researchers found that in mild COVID-19, CD8+ resident-memory (TRM) and CD4+ T-helper-17 (TH17) cells undergo an active (presumably antigen-driven) expansion towards the end of the monocyte-to-macrophage trajectory, and are characterized by good effector functions, while in critical COVID-19 they remain more naïve. Vice versa, CD4+ T-cells with T-helper-1 characteristics (TH1-like) and CD8+ T-cells expressing exhaustion markers (TEX-like) are enriched halfway their trajectories in mild COVID-19, where they also exhibit good effector functions, while in critical COVID-19 they show evidence of inflammation-associated stress at the end of their trajectories. Monocyte-to-macrophage trajectories show that chronic hyperinflammatory monocytes are enriched in critical COVID-19, while alveolar macrophages, otherwise characterized by anti-inflammatory and antigen-presenting characteristics, are depleted. In critical COVID-19, monocytes contribute to an ATP-purinergic signaling-inflammasome footprint that could enable COVID-19 associated fibrosis and worsen disease-severity. Finally, viral RNA-tracking reveals infected lung epithelial cells, and a significant proportion of neutrophils and macrophages that are involved in viral clearance.
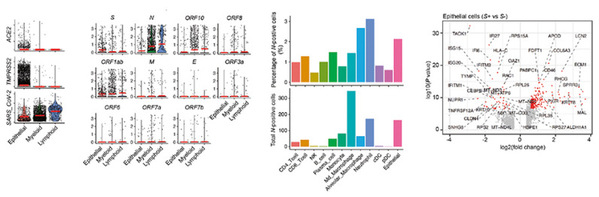
These findings bear crucial therapeutic relevance. The RECOVERY trial recently claimed that dexamethasone reduces deaths by one-third in hospitalized patients with critical COVID-19. Dexamethasone has indeed been shown to dampen myeloid inflammatory signaling (notably IL-1 and IL-6 release), reduce neutrophil inflammation, promote a macrophage phenotype with anti-inflammatory and phagocytic traits, and to maintain clonal balance in T-cells. Relevant data also suggest that neutrophils are key players in the acute phase of the infection. However, prolonged neutrophil inflammation might also cause excessive collateral lung damage and be detrimental to the host, as suggested by autopsy reports. In this regard, it remains to be confirmed whether immunomodulatory drugs that modulates the neutrophil function represent a promising therapy for COVID-19.