New mechanism of cancer escape from immunity via EGFR-GSK3α-ARIH1 signaling
Tumor is one of the major hazards for human health and life. With the cross-disciplinary fusion of immunology and cell biology, tumor immunotherapy technology has become an important means of anti-tumor treatment.
Programmed death ligand-1 (PD-L1) is constitutively expressed on the surface of cancer cells. The interaction between PD-L1 and its receptor, programmed death protein-1 (PD-1), which is mainly expressed on the surface of T cells, results in cancer cell evasion from immune surveillance. PD-1 and PD-L1 have thus become crucial immune checkpoint blockade immunotherapy targets for different types of cancers. Antibodies against Pd-1 or Pd-L1 have been approved by the U.S. Food and Drug Administration (FDA) for second-line or even first-line treatment of various cancers, such as non-small cell lung cancer, head and neck squamous cell carcinoma, renal cell carcinoma, melanoma and classic Hodgkin’s lymphoma. However, the clinical response rates for monoclonal antibodies are inconsistent and generally low, with some solid tumors having a response rate as low as 20%. Furthermore, these monoclonal antibodies are expensive and often unable to achieve expected therapeutic effects after dosing due to drug resistance.
In response to many flaws with anti-PD-1/ PD-L1 drugs, scientists have made every endeavor to develop small molecule drugs as an alternative to antibodies in order to improve efficacy, reduce costs and overcome resistance.
The research team led by XIA Hongguang from the Zhejiang University School of Medicine/ Liangzhu Laboratory published their latest findings in an open-access article entitled “ARIH1 Signaling Promotes Anti-tumor Immunity by Targeting PD-L1 for Proteasomal Degradation” in the journal Nature Communications on April 20. In this study, researchers screened out several compounds as inducers of PD-L1 degradation, identified ARIH1 as the E3 ubiquitin ligase responsible for targeting PD-L1 to degradation and delineated the mechanism of PD-L1 degradation and cancer escape from immunity via EGFR-GSK3α-ARIH1 signaling. This study suggests that ARIH1 might become a potential drug target to boost immunotherapy.
Xia’s research team has long been committed to research into protein degradation and drug development. In this study, they first screened a panel of 2125 FDA-approved drugs or drug candidates and found that ES-072, a third-generation EGFR inhibitor, induced a potent degradation of PD-L1. Their follow-up study suggests that inhibition of EGFR activates GSK3α by suppressing AKT activity, which can subsequently promote phosphorylation at the intracellular Ser279 and Ser283 residues of PD-L1, causing ARIH1-mediated ubiquitination and proteasome-mediated degradation.
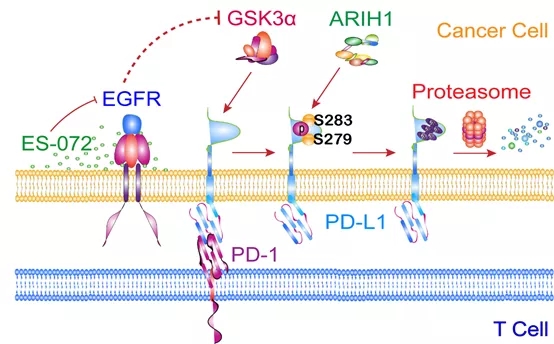
Schematic model for GSK3α-promoted and ARIH1-mediated PD-L1 degradation
4T1 is a mouse breast cancer cell line. Experiments revealed thatARIH1 overexpression had no effect on cell proliferation in vitro and on tumor growth in immune-deficient nude mice. However, researchers observed a dramatically suppressed tumor growth in the ARIH1-OE group in immunocompetent BALB/c mice, the majority of which exhibited a complete tumor regression. Meanwhile, the levels of total and activated CD8+ cytotoxic T cells (GzmB+) that infiltrated the tumor microenvironment increased significantly in the ARIH1-OE group. Considering that 4T1 cells are a well-known cold tumor and that PD1-PDL1 antibodies alone have literally no inhibitory effect on 4T1 tumors, this study also indicated that ARIH1, which can not only regulate PD-L1 degradation but also reshape the tumor microenvironment, may well be a key molecule to turn “cold tumors” into “hot tumors”. The triple-negative breast cancer is also a famous “cold tumor”, but clinical trials with PD-1 antibodies have failed. “Our research suggests that ARIH1-activating agents are expected to become drug targets for a series of cold tumors, including the triple-negative breast cancer,” XIA Hongguang said.