Researchers discover a novel mechanism for reversing anti-PD-L1 resistance in liver cancer
China has the highest incidence of liver cancer in the world. For patients with early liver cancer, surgery is the optimal treatment, but most patients are diagnosed with advanced liver cancer and thus miss the opportunity for surgery. Due to the limitations of targeted drugs Sorafenib and Regorafenib, the immune “brake-point” drug represented by the anti-PD-1/PD-L1 axis holds promise for curing patients with advanced liver cancer. At present, Opdivo (Nivolumab), a drug for the PD-L1/PD-1 axis approved by the FDA, entered the Chinese market in June 2018 and achieved good results in various tumor treatments. However, liver cancer is characterized as rich in immunosuppressive cell infiltration, and the anti-PD-1/PD-L1 treatment is not effective. Preclinical studies reveal that the response rate of the anti-PD-1/PD-L1 treatment adds up to little more than 14.3%. Therefore, it is a top priority to reverse this situation, explore new strategies for fighting liver cancer, and improve prognosis in patients.

Tumor-associated macrophages (TAMs) are recognized as antitumor suppressor, but how TAMs behave in the hypoxic environment of hepatocellular carcinoma (HCC) remains elusive.The research team led by CAE fellow ZHENG Shusen elaborated on the finding that hypoxia acts as a catalyst for anti-PD-L1 resistance in liver cancer. Hypoxia accelerates tumor cell proliferation and matrix remodeling via hypoxia inducible factor 1α (HIF-1α), which regulates the transcription of a variety of oncogenes upon oxygen deficiency. ZHENG et al. discovered that hypoxia inducible factor 1α (HIF-1α) induced increased expression of triggering receptor expressed on myeloid cells-1 (TREM-1) in TAMs, resulting in immunosuppression.
Specifically, TREM‐1+ TAMs were abundant at the advanced stages of HCC progression, which indirectly impaired the cytotoxic functions of CD8+T cells and induced CD8+T cells apoptosis. Biological and functional assays demonstrated that TREM‐1+ TAMs had a higher expression of PD‐L1 in the hypoxic environment. However, TREM‐1+ TAMs could put an end to spontaneous and PD‐L1‐blockade‐mediated antitumor effects in vivo, suggesting that TREM‐1+ TAM‐induced immunosuppression was dependent on a pathway separate from the PD‐L1/PD‐1 axis. Moreover, TREM‐1+ TAM‐associated Tregs were crucial for HCC resistance to anti‐PD‐L1 therapy. Mechanistically, TREM‐1+TAMs elevated CCL20 expression through the ERK/NF‐κβ pathway in response to hypoxia and tumor metabolites leading to CCR6+Foxp3+ Treg accumulation. Blocking the TREM‐1 pathway could significantly inhibit tumor progression, reduce CCR6+Foxp3+ Treg recruitment and improve the therapeutic efficacy of PD‐L1 blockade.
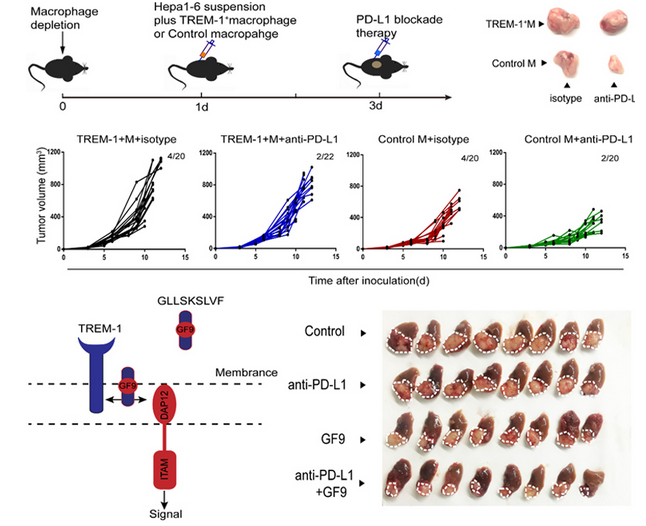
Thus, these data revealed that blocking TREM-1+ TAMs with GF9 offered a promising therapeutic strategy to overcome the resistance to anti-PD-L1 therapy in HCC.
This ground-breaking discovery is published in an article entitled “Blocking TREM-1+ Tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-PD-L1 resistance in liver cancer” in the journal of HEPATOLOGY.