A microrobot developed for tumor-targeting therapy via photosynthesis
Microrobots are simply microscopic-scale automated machines designed to perform selected movements in response to specific stimuli—but their tiny size means that they could travel through the body to perform tasks that no conventional robot could do. Recent advances in microrobotics have exhibited considerable biomedical potential for targeted delivery, disease monitoring and minimally invasive surgery. Up till now, most of the propelled microrobots have been applied in vitro. However, more envisioned functionalities of the microrobots aimed at in vivo treatments remain to be appreciably challenging.
Recently, the research team led by Prof. ZHOU Min from the Zhejiang University Second Affiliated Hospital and the Institute of Translational Medicine developed a photosynthetic biohybrid nanoswimmers system (PBNs), magnetic S. platensis (MSP) hybrid microswimmers. This work provides a novel platform for achieving tumor-specific enhanced combination therapy under the guidance of fluorescence (FL)/photoacoustic (PA)/MR imaging.
This study is published as a cover article in the journal of Advanced Functional Materials.
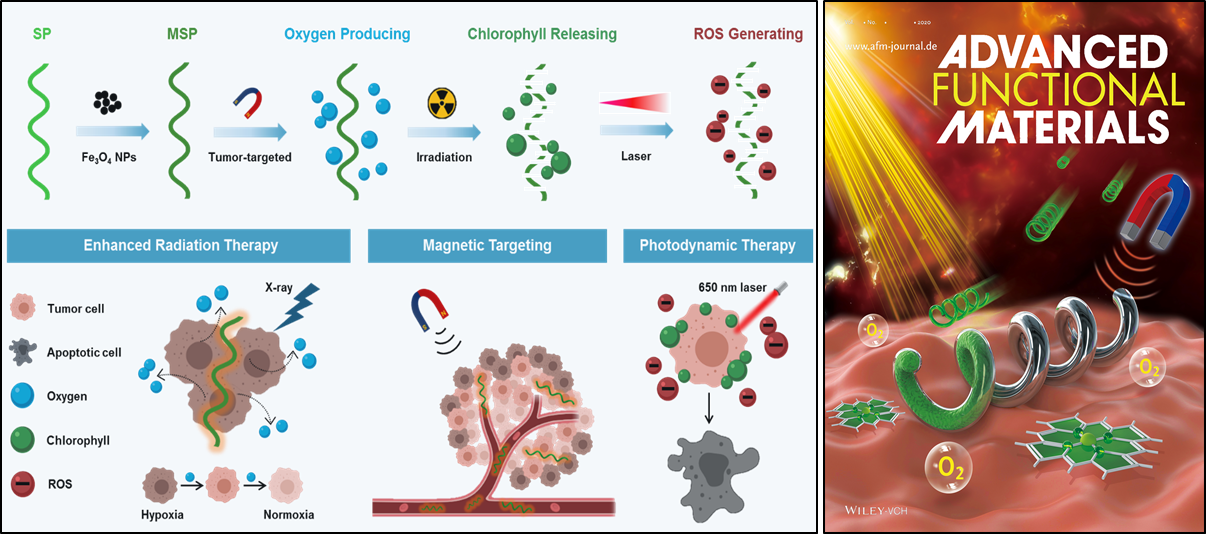
The tumor microenvironment (TME), marked by tumor hypoxia, is largely responsible for the resistance of tumors to many conventional cancer therapies. For oxygen-dependent therapies such as radiotherapy (RT) and photodynamic therapy (PDT), the therapeutic effects are severely circumscribed by the hypoxic tumor microenvironment and the rapid consumption of oxygen.
S. platensis is one kind of most well-sourced natural photosynthetic microalgae because of its biomass abundance in the natural environment. It not only provides an efficient and accurate model for 3D locomotion but also functions as a photosensitizer for photodynamic therapy and a theranostic agent for FL and PA imaging. To fabricate biohybrid microswimmers, S. platensis is used as an actuator and functionalized with superparamagnetic Fe3O4 nanoparticles (NPs), which are utilized for both precise directional locomotion and magnetic resonance (MR) imaging contrast agent.
“The innovation of this study lies in the hybrid microswimmer capable of selectively delivering drugs to the hypoxic tumor site,” ZHOU Min observes, “This system can not only generate oxygen efficiently thanks to S. platensis but also have the magnetically-targeting function due to NPs.”
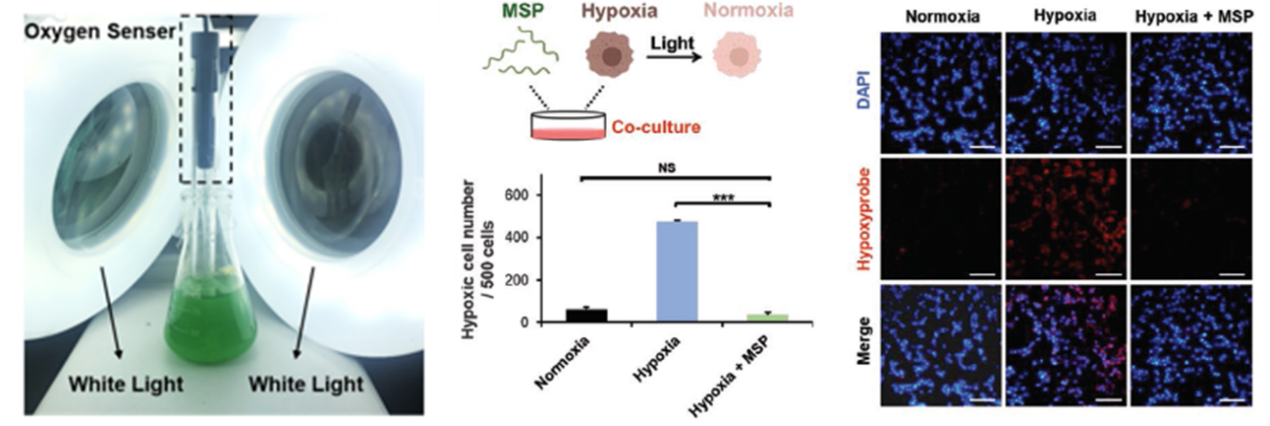
In the process of treatment, the PBNs can be used as an oxygenerator in hypoxic solid tumors through photosynthesis to modulate the tumor microenvironment, thus improving the effectiveness of RT. “The in vitro enhanced therapy plays a salient role in inhibiting the growth of tumors in mice with breast cancer cells.”
Furthermore, the innate chlorophyll released from the RT-treated PBNs, as a photosensitizer, can produce cytotoxic reactive oxygen species under laser irradiation to achieve photodynamic therapy. The PBNs is also well-equipped with the functions of chlorophyll-based fluorescence and photoacoustic imaging, which can monitor the tumor therapy and the tumor TME environment.
Thanks to these remarkable properties, the PBNs can be used as a tumor-targeting oxygenerator for in situ oxygen generation to regulate the hypoxic tumor microenvironment for FL/PA/MR imaging-guided diagnosis and the synergistic RT/PDT treatment of tumors.