ZJU scientists open the door to the regulatory mechanisms in the brain
The G protein-coupled receptor (GPCR) is also known as seven-(pass)-transmembrane domain receptor in that it passes through the cell membrane seven times. It is involved in virtually any physiological and pathological process. As a major drug target, approximately 40% of the drugs take effect through the GPCR. Metabotropic γ-amino butyric acid (GABAB) receptor, located in the central nervous system, mediates slow and prolonged inhibitory activity. Dysfunctions of the GABAB receptor or mutation in its genes have been implicated in a variety of neurological and psychiatric disorders, including epilepsy, anxiety, depression, schizophrenia and drug addiction.
However, the discovery of target drugs is a formidable and thorny task due primarily to the fact that candidate drugs cannot pinpoint the GABAB receptor, thereby failing to measure up to expectations and giving rise to side effects. Moreover, lack of the high-resolution 3D structure of the class C GPCR also poses a tremendous challenge for the screening and designing of specific drugs.
Recently, the research team led by Prof. ZHANG Yan from the Department of Biophysics in the Zhejiang University School of Medicine conducted relevant research in collaboration with the research team led by Prof. LIU Jianfeng from the School of Life Science and Technology at Huazhong University of Science and Technology. Their research findings are published in the June issue of Cell Research. It is the first time that the precise cryogenic electron microscopy (cryo-EM) structure of the full-length human heterodimeric GABAB receptor has been presented.
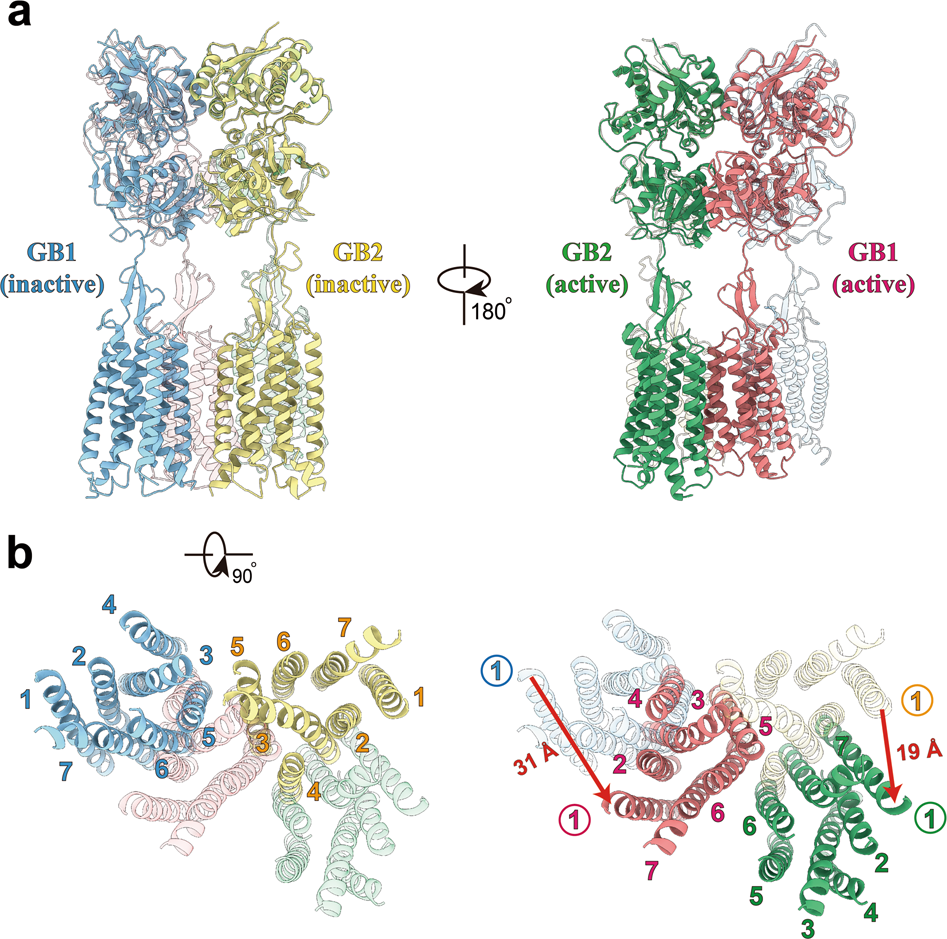
a, b Orthogonal views of the superimposed structures of GABAB receptor in inactive and active states, showing the domain repositioning upon agonist binding-induced activation. Side views (a) and intracellular views (b) of superposed structures are shown, with the active structure in translucent in the left panels and the inactive structure in translucent in the right panels, respectively. VFT domains and loops are omitted for clarity in b. Red arrows indicate the translation direction and distance for GB1 and GB2 (measured at extracellular tips of TM1 helices), respectively. Structures were aligned on the combined domains of GB1 VFT and GB2 lobe 1, the relatively stable parts of the receptor along activation pathway.
An interesting analogy can be drawn between a human body and a building. Cells can be likened to rooms and GPCRs can be compared to various doors used to convey information. The heterotrimeric G protein is a happy family. As long as the right key (the moderator of the GPCR) is available, one can walk through the door and lead a rich and colorful life. In the human body, there are a diversity of similar “doors” through which cells can receive a body of external information and play different roles.
As “commander-in-chief” of the body, the brain needs to stay in a moderately active state. If it is excessively excited or depressed, there will be negative consequences. How can the brain stay moderately excited? The GABAB receptor can effectively mediate inhibitory neurotransmission in the central nervous system so that the brain will keep moderately “calm”.
ZJU researchers identified the high-resolution structure of the GABAB receptor in both inactive and active states via cryo-EM) They discovered that the GABAB receptor has two subunits, each composed of the extracellular Venus flytrap (VFT) domain and the transmembrane (TM) domain. The VFT domain is responsible for receiving external stimuli while the TM domain passes signals to cells.
Researchers also unexpectedly discovered that the GABAB receptor has a specific doorway at the bottom. “Such a doorway is quite common in some homes, making it easy for pets to enter and exit and for letters to be delivered.” This feature enables a drug to recognize the GABAB receptor “at a glance”.
Even if the feature of the “door” is identified, lack of knowledge about how to open it still affects signal transmission.
“It’s like a locked door. You need to find the right key to the door from thousands of keys, which involves definitely a herculean task taking massive amounts of time and money. However, if the structure of the lock cylinder is presented, chances are that one can figure out the configuration of the key, thereby saving a lot of time,” MAO Chunyou, the lead author of the research and a postdoctoral fellow in the School of Medicine.
“We are very proud that the structural resolution of our analysis is second to none in the world,” SHEN Cangsong added, “Scientists can obtain highly specific drug candidates on the strength of high-resolution structures or computer-assisted virtual drug screening techniques.”
ZJU researchers revealed a novel activation mechanism for the GPCR. They found that the left door received an upstream drug signal, which was passed downstream through the right door.
“Structural pharmacology holds immense promise,” ZHANG Yan remarked.
COVID-19 is currently rampant all over the world, jeopardizing human life and health. RNA polymerase, which is central to the self-replication and rapid infection of the novel coronavirus, is the target for nucleosides such as Remdesivir.
In early April, ZHANG Yan cooperated with scientists from the CAS Shanghai Institute of Materia Medica and the Chinese Academy of Medical Sciences in analyzing the cryo-EM structures of Remdesivir and the RNA-dependent RNA polymerase. This study revealed the structural basis of the anti-virus activity of Remdesivir, thus offering precise 3D structures for nucleoside target drugs. Relevant findings were published in the journal of Science on May 1.
“Identifying the full-length structure of the important protein of the virus is of appreciable significance to fabricating effective drugs with minimal side effects in a precise way,” said ZHANG Yan.
