Scientists discover the role of ANG in inhibiting intestinal inflammation
Recently, the research team led by XU Zhengping and SHENG Jinghao from the Zhejiang University School of Medicine discovered that ANG can maintain gut microbial homeostasis by balancing α-Proteobacteria and Lachnospiraceae. Their findings are published in the journal of GUT.
Everyone has experienced stomach aches, diarrhea and losses of appetite that make him or her truly feel intestinal discomfort and want to have a healthy intestine. Unfortunately, the incidence of a digestive disease called inflammatory bower disease (IBD) has been on the rise these years. IBD is characterized by chronic inflammation of the digestive tract with such symptoms as recurring diarrhea, abdominal pain and rectal bleeding. With the gradual progression of IBD, the structure and function of the digestive tract will be severely impaired. At present, it is believed that genetic susceptibility genes and a multitude of environmental factors contribute to the development of IBD, but its specific pathogenesis remains obscure. Researchers from various countries are committed to delving into the pathogenesis of IBD and finding effective approaches to preventing, diagnosing and treating IBD. A massive body of studies proves that intestinal microbes play a crucial role in the development of IBD.
Gut microbiota are the microorganisms that live in the digestive tracts of humans. Although we tend to neglect the existence of gut microbiota, they do indeed play a vital role in the well-being of human beings. Gut microbiota, as a diverse and dynamic bacterial community, are instrumental to digestion, differentiation of intestinal cells, protection of the host from pathogen invasion, and stimulation and regulation of the immune system. However, affected by internal and external factors, gut microbiota may well become structurally and/or functionally dysfunctional. In this case, a variety of disorders may be induced by disrupted intestinal microbiota.
The immune system participates in the organization of a healthy host–microbe interface through production of IgA, secretion of mucus and induction of antimicrobial peptides (AMPs). AMPs are produced by the host during their symbiotic interaction with microbiota. An important function of AMPs is to establish the host–microbe homeostasis rather than to eliminate the microbial symbiont population. Currently, it is recognized that AMPs display multiple biological activities and influence the host–microbe interplay through various mechanisms, such as direct killing of bacterium by disrupting its cell membrane, prevention of bacterial invasion by suppressing motility of flagellated bacterium or modulation of innate immune response.
Angiogenin (ANG), originally isolated as a tumor angiogenic factor, is also identified as an AMP. ANG exhibits broad-spectrum antimicrobial activities against bacteria and fungi by disrupting cell membranes or by degrading cellular RNAs as demonstrated in vitro. However, the in vivo role of ANG in intestinal microbiota and its contribution to gut health remain elusive.
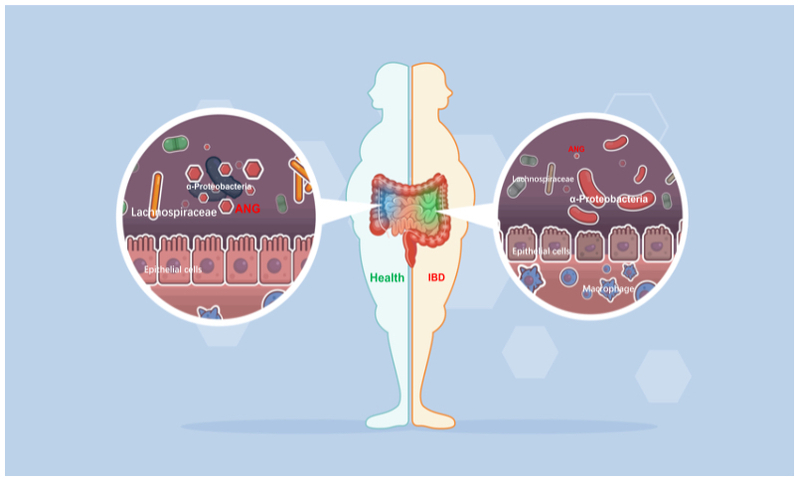
The researchers from ZJU evaluated the effect of ANG on microbiota and its contribution to colitis in different colitis models with co-housing and faecal microbiota transplantation. ANG-regulated bacteria were determined by 16S rDNA sequencing and their functions in colitis were analyzed by bacterial colonization. The species-specific antimicrobial activity of ANG and its underlying mechanism were further investigated with microbiological and biochemical methods.
The researchers discovered that ANG could regulate microbiota composition and inhibit intestinal inflammation. Specifically, Ang1 deficiency in mice led to a decrease in the protective gut commensal strains of Lachnospiraceae but an increase in the colitogenic strains of α-Proteobacteria. Direct binding of ANG to α-Proteobacteria resulted in lethal disruption of bacterial membrane integrity, and consequently promoted the growth of Lachnospiraceae, which would otherwise have been antagonized by α-Proteobacteria. Oral administration of ANG1 reversed the dysbiosis and attenuated the severity of colitis in Ang1-deficient mice.
This research demonstrates a brand-new role of ANG in shaping gut microbe composition and maintaining gut health, suggesting that the ANG-microbiota axis could be developed as a potential preventive and/or therapeutic approach to dysbiosis-related gut diseases.