Converting propane into propene is expected to be more affordable and efficient
The global pandemic has brought in its wake a steep rise in the consumption of face masks and an acute shortage of their raw material—propene. In 2020, the consumption of propene added up to 116 million tons globally. To meet the increasing demands for propene, oxidative dehydrogenation of propane (ODHP) has emerged as one of the key industrial technologies. In this regard, researchers, including XIAO Feng-Shou and WANG Liang from the Zhejiang University College of Chemical and Biological Engineering and MENG Xiangju from the Zhejiang University Department of Chemistry developed a more active and durable catalyst which is expected to make propene production more affordable and efficient. Their study was published in an open-access article entitled “Isolated boron in zeolite for oxidative dehydrogenation of propane” in the journal Science on April 2.
Propene has been extensively used as a building unit for the production of plastics, fibers, and intermediates for the synthesis of fine chemicals. The current method for propene production relies predominantly on the steam cracking of heavy hydrocarbons in naphtha. It is noteworthy that there are two routes: nonoxididative dehydrogenation of propane and ODHP. The former is primarily adopted at present, but it has insufficient lifetimes because of thermodynamically favorable coke formation and metal sintering, which require frequent catalyst regeneration.
By contrast, ODHP has substantial advantages in terms of exothermic features and hindrance of coke formation. There has been a body of research into ODHP in academia, but it remains to be a challenge for practical applications for lack of an appropriate catalyst.
To satisfy industrial needs, researchers tend to go back to the lab and seek solutions at the scientific level. “A catalyst with remarkable application prospects should be marked by superb selectivity, high catalytic activity and outstanding durability. Among these ingredients, selectivity is a top priority, and it is also the biggest challenge in ODHP. Due to the active nature of propene, it may well be easily over-oxidized, thus causing the poor selectivity of the catalyst,” said XIAO Feng-Shou.
As early as 2016, hope was ignited. I. Hermans from the University of Wisconsin and LU Anhui from Dalian University of Technology discovered the remarkable selectivity of boron nitride in oxidative dehydrogenation. This research sparked enthusiasm in the academic community, but the wave of passion was soon extinguished. Studies revealed that although boron nitride has excellent selectivity, its catalytic activity and water-resistant stability fail to measure up to the actual needs. Therefore, there was a unanimously negative view that the catalytic activity of boron-containing catalysts originates from multiple centers and that isolated boron cannot work.
XIAO Feng-Shou and WANG Liang reentered this legendary “dead end” to find out the true story: Where is the active site of the boron-based catalyst and how does catalytic activity work? To this end, researchers developed a zeolite molecular sieve catalytic material centered on isolated boron.
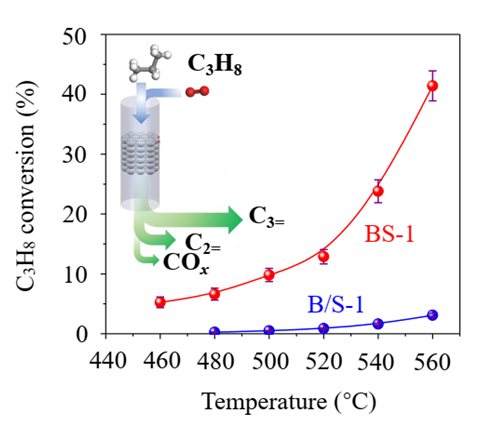
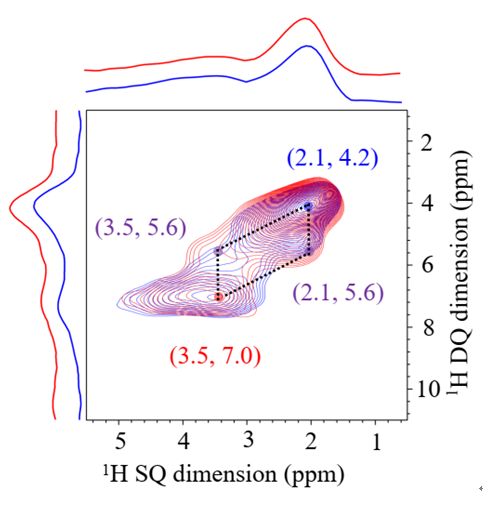
“In addition to the active site itself, the ‘environment’ in which it is located is also essential to the design of a catalyst,” said WANG Liang, “In other words, its ‘neighbors’ and the layout are equally important.” Researches used a type of zeolite molecular sieve material with a more common structure. In this structure, isolated boron is surrounded by silicate species. Much to their surprise, this isolated boron with a specific structure in borosilicate zeolite as an active center exhibits remarkable catalytic performance.
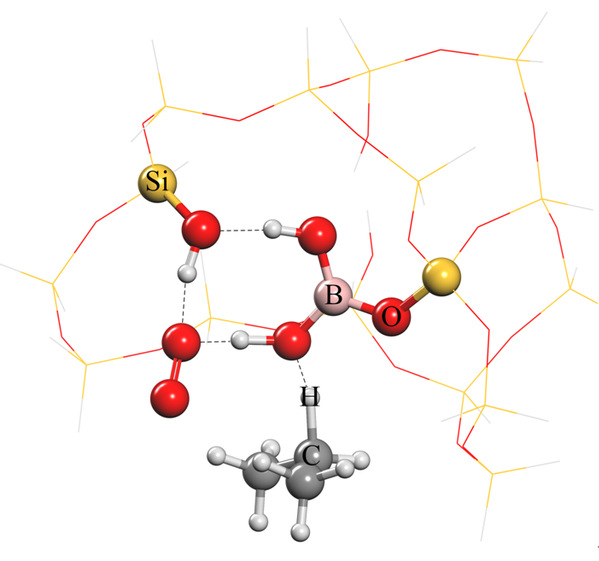
Researchers further explained the reason for impressive catalytic performance. In the dehydrogenation reaction of propane, boron hydroxyl groups and one of the silicon hydroxyl groups can activate propane and oxygen molecules simultaneously to form a stable intermediate which can further convert into propene. Furthermore, the reversible hydrolysis-condensation process of Si-O-B bonds can effectively hinder the formation of water-soluble boric acid and thus achieve superb stability.
In a continuous 220-hour “endurance” test, this zeolite-catalytic oxidative dehydrogenation process maintained a high selectivity of 83% and achieved a conversion rate of 32.9-43.7%. This work breaks conventional knowledge that isolated boron centers cannot catalyze propane dehydrogenation reactions and further deepens the understanding of propane dehydrogenation and its active centers. It represents a large stride forward in boron catalysis that may lead the way to scalable applications in ODHP.