ZJU scientists explain regulation of extracellular vesicles by dephosphorylating Syntenin
On March 10, the research team led by Prof. KE Yuehai at the Zhejiang University School of Medicine published an open-access article entitled “Phosphatase Shp2 regulates biogenesis of small extracellular vesicles by dephosphorylating Syntenin” in Journal of Extracellular Vesicles. This study identified a group of tyrosine phosphatases that regulate the synthesis and secretion of small extracellular vesicles (sEVs) and elucidated that tyrosine phosphatase PTPN11/Shp2 negatively controls the biogenesis of exosomes in epithelial cells by dephosphorylation. It suggested a new pathway for phosphatase involvement in the microenvironment of lung inflammation.
Exosomes are a major form of extracellular vesicles, ranging from 20 nm to 200 nm in size. Recent studies have shown that exosomes are the main messenger for cell-to-cell communication and under physiological or pathological conditions, the synthesis and release of exosomes can mediate the transmission of various bioactive molecules, which are actively involved in the regulation of disease occurrences. Analyzing the regulatory mechanism of exosomes helps provide insight into the common features of signal transduction in the complex microenvironment.
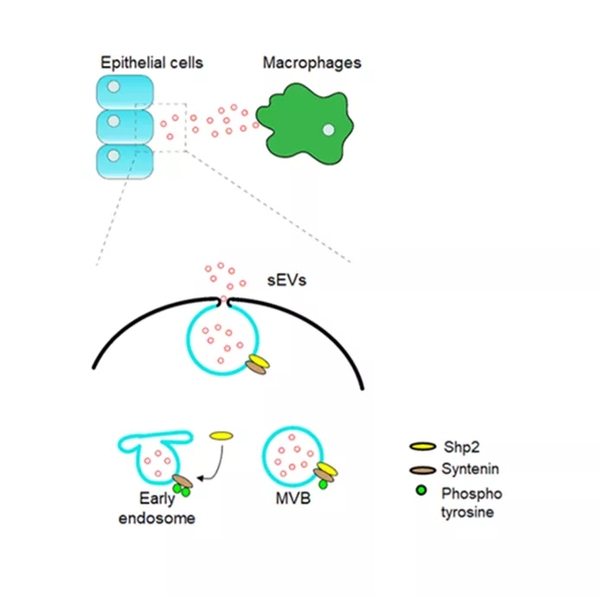
Phosphorylation is a crucial post-translation mode for signal regulation, the biochemical basis for the understanding of molecular mechanisms, and a key target for drug intervention, of which tyrosine phosphorylation has captured immense attention. This study revealed that the loss or inhibition of PTPN11/Shp2, a type of non-receptor tyrosine phosphatase, can significantly enhance the secretion of exosomes in various cells. By Co‐immunoprecipitation (Co‐IP) and in vitro dephosphorylation assays, researchers identified that Shp2 negatively controlled sEV biogenesis by directly dephosphorylating tyrosine 46 of Syntenin, thereby affecting the Syntenin‐ALIX complex and controlling the formation of the endosomal sorting complex required for transport (ESCRT). This study also found that dephosphorylation plays a role in controlling the synthesis and secretion of sEVs, and that several kinds of tyrosine phosphatases are involved in this process, among which Shp2 is one of the key pathways. Meanwhile, this study indicated that Shp2 dysfunction leads to enhanced epithelial sEV generation in vitro and in vivo. The increase in epithelial sEVs caused by shRNA‐mediated down‐regulation of Shp2 promotes macrophage activation, resulting in strengthened inflammation.
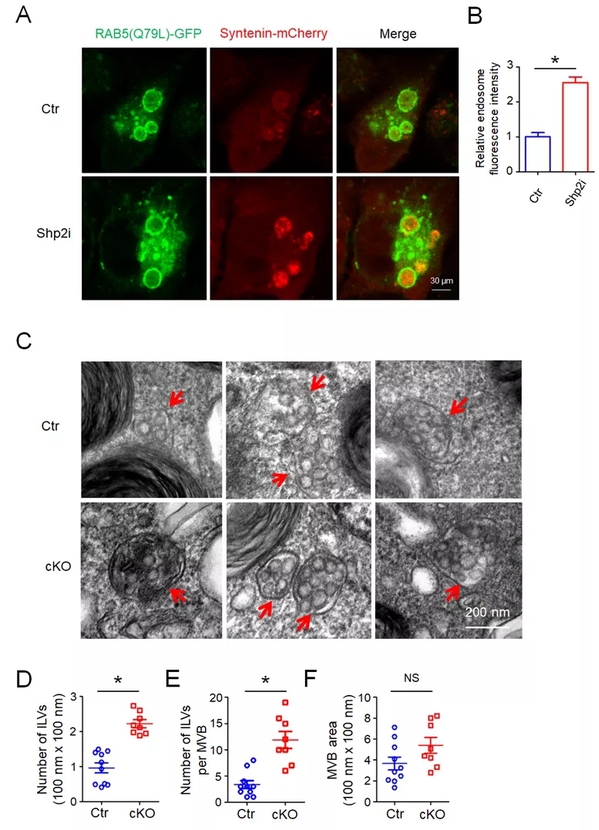
“These findings provide a basis for understanding the mechanism of sEV formation and relevant function in epithelial‐macrophage crosstalk,” said KE Yuehai.