How cancer cells grow and survive in harsh microenvironments
Rapid tumor growth inevitably results in insufficient nutrient supply in tumor microenvironment. To prepare for the challenge arisen from energy stress, tumor cells synthesize and store lipid in a format of lipid droplet as important energy resources when nutrients are sufficient.
Lipid droplets, spherical organelles in the cytosol, are composed of a neutral lipid core consisting mainly of triacylglycerols and sterol esters surrounded by a phospholipid monolayer. Lipid droplets are coated with proteins, such as the perilipin protein family members perilipin (PLIN)2 and PLIN3. Also, the lipid in lipid droplets can be used for energy production via fatty acid oxidation, membrane biogenesis for cell growth, protein modification, signaling, and secretion with lipoproteins.
The research team led by Dr. LU Zhimin from Zhejiang University Institute of Translational Medicine published an article entitled “Choline kinase alpha 2 acts as a protein kinase to promote lipolysis of lipid droplets” in Molecular cell on May 25, 2021. This study unravels a critical mechanism underlying the process of lipolysis of lipid droplets for survival and growth of tumor cells under energy stress conditions.
Tumor cells have highly active lipid metabolism reflected by increased lipid synthesis and dynamic lipolysis. In 2020, Dr. LU’s lab discovered a novel mechanism underlying aberrant activation of lipid anabolism in tumor cells. Phosphoenolpyruvate carboxykinase 1 (PCK1), a key enzyme in the gluconeogenesis pathway, functions as a protein kinase to activate transcription factor SREBP for lipid synthesis, leading to enhanced accumulation of lipid droplets in tumor cells (Nature, PMID: 32322062). Nevertheless, how cancer cells initiate lipid droplet lipolysis in response to energy stress remains unknown.
In the current report, Dr. Lu 's lab demonstrated that glucose deprivation induced a series of modifications of CHKa2 including phosphorylation and acetylation, which converted CHKa2 dimer to its monomers with altered catalytic domain conformation and exposed a binding site for interaction with PLIN2/3. Importantly, CHKa2 functioned as a protein kinase and phosphorylated PLIN2 at Y232 and PLIN3 at Y251. This phosphorylation promoted lysosomal degradation of PLIN2/3 mediated by chaperone (HSC70)-mediated autophagy (CMA), thereby enabling lipolysis of lipid droplet by cytosolic lipase and autophagosome to promote tumor cell survival and brain tumor growth.
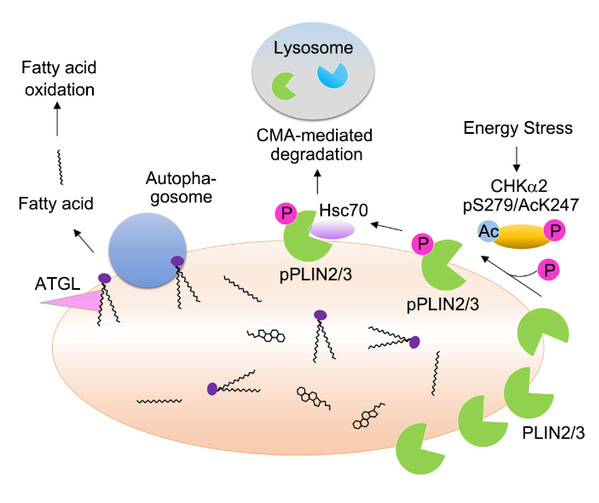
The clinical significance of the reported regulation was exhibited by the positive correlation of PLIN2 Y232 and PLIN3 Y251 with poor prognosis of glioblastoma patients, indicating that the phosphorylation levels of these proteins can be a biomarker for glioblastoma aggrievances.
“These findings highlight the role of CHKa2 as a protein kinase in lipolysis and glioblastoma development and provide a strategy for development of drugs targeting tumor lipid metabolism”, said Dr. LU.
Source: School of Medicine