ZJU oncologists characterize a genomic landscape of alpha-fetoprotein producing gastric cancer
Being the fifth morbidity and the third leading cause of tumor mortality worldwide, gastric cancer (GC) remains to be a major health burden. Although alpha-fetoprotein (AFP) has been widely applied as a relatively specific marker for liver cancer, some other tumors such as a subgroup of gastric cancer can also produce and secrete AFP, namely AFP producing gastric cancer (AFPGC). AFPGC is a special and rare subtype of gastric cancer, accounting for 1.3-5.4% of GC. Compared with common GC, patients with AFPGC have a higher rate of liver metastasis and worse prognosis.
So far, the molecular features and effective therapy for AFPGC remain a mystery. The research team led by Dr. TENG Lisong from the First Affiliated Hospital of Zhejiang University School of Medicine retrospectively analyzed 105 AFPGC and conducted whole-exome sequencing (WES) on 58 cases, and characterized a landscape of genomic characteristics of AFPGC. Meanwhile, an ideal platform of patient-derived xenogragt (PDX) models was established to explore and validate the genome-guided personalized treatment for this disease. This study was published in Nature Communications on June 24, 2021.
Through bioinformatic analysis of the largest next-generation sequencing cohort of AFPGC, the current study revealed the specific somatic mutation spectrum, copy number variations, and key altered pathways of AFPGC. Comparative analyses revealed that AFPGC has distinct genomic features from gastric cancer of the Cancer Genome Atlas as well as four molecular subtypes. Further analysis revealed that AFPGC with amplification of CCNE1 at 19q12 and/or ERBB2 at 17q12 show poorer survival and more aggressive, while this phenomenon was absent in common GC. These findings elucidated the unique molecular properties and provided a rationale for the development of specialized treatment.

The landscape of somatic mutations in AFPGC
Based on the frequent genomic alterations of AFPGC, translational research was performed and the therapeutic value of targeting CCNE1 and ERBB2 was validated to explore the precise therapeutic target for this disease. By establishing a large collection of AFPGC patient-derived xenograft (PDX) models, the researchers found dual inhibition of ERBB2 and CCNE1 has a synergistic antitumor effect, which could act as a potential treatment strategy to overcome or reverse the potential treatment resistance.
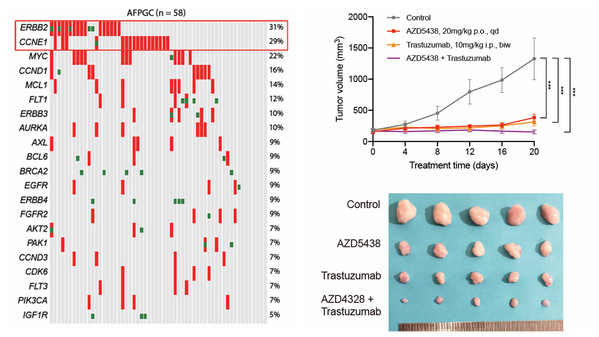
Potential targetable genes and PDX model of AFPGC
“Precise treatment has been a promising area for cancer therapy. For this rare subtype of GC, one-size-fits-all strategy has been unsatisfactory in clinical practice. Our study provided an understanding of genomic characteristics of AFPGC and propose a platform to explore and validate the genome-guided personalized treatment for this disease.” Dr. TENG said.
Source: School of Medicine