ZJU scientists discover the molecular mechanism of RBSDV inducing cell autophagy in Laodelphax striatellus
Rice viruses are prevalent in many rice-growing countries and often cause serious damages to rice production. Among them, the rice black-streaked dwarf virus (RBSDV), transmitted by the small brown planthopper Laodelphax striatellus, causes tremendous losses in China’s grain yields every year. Therefore, discovering the transmission mechanism of RBSDV is of immense significance for its effective control.
The research team led by Prof. WU Jianxiang and Prof. ZHOU Xueping from the Zhejiang University College of Agriculture and Biotechnology published an open-access article entitled “Rice black-streaked dwarf virus P10 promotes phosphorylation of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) to induce autophagy in L. striatellus” in the journal Autophagy.
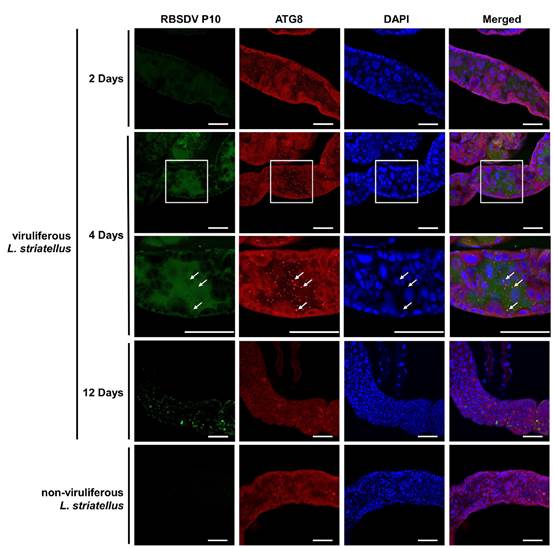
RBSDV infection-induced autophagy in L. striatellus midguts at various days post-feeding.
The research team discovered that the early phase of RBSDV infection in L. striatellus can induce autophagy, leading to the suppression of RBSDV invasion and accumulation while inhibiting autophagy can promote RBSDV invasion and accumulation and thus improve the mortality rate of RBSDV-infected L. striatellus. This indicates that autophagy, as an innate immune response, plays a crucial role in the battle against RBSDV invasion. Furthermore, the main capsid protein (also known as P10) of RBSDV alone can induce autophagy in both Sf9 and L. striatellus cells. Yeast two-hybrid (Y2H), pull down, Co-IP assays confirmed that RBSDV P10 can interact with GAPDH in vivo and in vitro. Further experiments indicated that Sf9 cells expressing RBSDV P10 can promote the phosphorylation of AMP-activated protein kinase (AMPK), resulting in GAPDH phosphorylation and relocation of GAPDH from the cytoplasm to the nucleus. Meanwhile, RBSDV invasion or feeding recombinant expressed RBSDV P10 can also promote LsAMPK phosphorylation, leading to LsGAPDH phosphorylation and the translocation of the phosphorylated LsGAPDH from the cytoplasm to the nucleus to activate the autophagy pathway in L. striatellus. Co-IP and in vitro phosphorylation assays showed that AMPK interacts with GAPDH, phosphorylated AMPK can phosphorylate GAPDH, and silencing AMPK genes can inhibit the occurrence of GAPDH phosphorylation, translocation of GAPDH into the nucleus and autophagy.
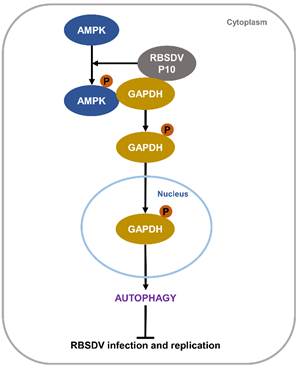
A working model of RBSDV P10-induced autophagy in L. striatellus cells.
This study reveals that RBSDV invasion or RBSDV P10 can induce AMPK phosphorylation, which can lead to GAPDH phosphorylation and the translocation of phosphorylated GAPDH into the nucleus. Once inside the nucleus, phosphorylated GAPDH can activate autophagy to suppress virus infection. “Our research illuminates the mechanism by which RBSDV induces autophagy in L. striatellus, and indicates that the autophagy pathway in an insect vector participates in the anti-RBSDV innate immune response,” said Prof. Wu. “This will provide new insights into RBSDV control.”