Novel mechanisms for establishment of intratumoral heterogeneity and drug-resistance in SCLC
Small cell lung cancer (SCLC), classified as a high-grade neuroendocrine (NE) tumor, accounts for approximately 15% of all lung cancers. It is a particularly aggressive and deadly form of lung cancer characterized by fast growth, early metastasis, and rapidly acquired therapeutic resistance. The overall 5-year survival rate of advanced-stage disease has remained at 1 to 5% for decades. The standard chemotherapy for SCLC consists of platinum-based chemotherapy combined with the topoisomerase II inhibitor etoposide. A remarkable response to the chemotherapy is followed almost invariably by the development of resistant disease.
Although most SCLC tumor cells display strong NE features, some tumor cells do not express NE genes and are so-called non-neuroendocrine (Non-NE) tumor cells. Emerging evidence indicates that the activation of Ras or Notch signaling within SCLC tumors promotes the generation of Non-NE SCLC cells. However, how the intratumor heterogeneity in SCLC tumors is established and pertains to the acquired therapeutic resistance and metastatic progression remains elusive.
On October 1, Prof. SONG Hai and Prof. SHEN Li et al. published an article entitled “YAP drives fate conversion and chemoresistance of small cell lung cancer” in the journal Science Advances, providing a theoretical basis for the treatment of SCLC.
In various tumors, the expression or activation of YAP/TAZ, the key transcriptional regulator in the Hippo pathways, induces tumorigenesis and progression. However, in SCLC, YAP/TAZ expression was not detected in more than 90% of tumor samples or NE tumor cell lines. Moreover, the degree of YAP/TAZ expression was negatively correlated with the expression of NE markers. To reveal the role of YAP in SCLC, researchers utilized single cell sequencing and immunohistochemistry to analyze a mouse SCLC model after the cell type-specific inactivation of Trp53, Rb1, and Pten in pulmonary NE cells (PNECs). They found that all tumor cells showed NE properties at the onset of SCLC. However, as the tumor progressed, Non-Ne tumor cells gradually appeared, and the proportion increased with the progress of the tumor. In follow-up experiments, researchers constructed a mouse SCLC model subjected to tetracycline-induced YAP expression and discovered that activation of YAP in SCLC could significantly suppress NE gene expression in SCLC and contributed to the transdifferentiation of NE tumor cells into Non-NE ones. They also found that YAP expression in SCLC could significantly activate the Notch signaling pathway. In addition, YAP could inhibit the NE properties of SCLC by activating the Notch signaling pathway and the expression of REST/NRSF, thereby inducing the transdifferentiation of NE cells into Non-NE ones.
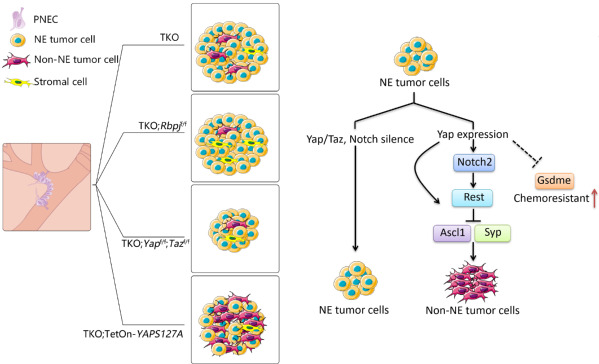
The model shows that YAP/TAZ regulate intertumoral heterogeneity and drug resistance of SCLC.
Researchers also found that YAP-activated tumor cells enhanced the resistance to chemotherapy drugs cisplatin and etoposide. Non-NE tumor cells were more resistant to chemotherapeutic drugs than NE counterparts. The difference was caused by the different death patterns of the two cell types, with Non-NE tumor cells and NE ones undergoing classical apoptosis and GSDME-mediated pyroptosis respectively. Further studies showed that activation of YAP in SCLC significantly inhibited the expression of GSDME, thereby suppressing the onset of scorch death and making SCLC more resistant to drugs.
“This study demonstrated that YAP plays critical roles in the establishment of intratumoral heterogeneity and highlights the potential of targeting YAP for chemoresistant SCLC,” said Prof. Song.