Licensing but not fueling: a metabolic enzyme promotes mitosis beyond metabolism
Uncontrolled cell proliferation is a hallmark of cancer. To sustain the ceaseless cell division, cancer cells rewire metabolic pathways in order to provide sufficient nutrient and energy for mitotic progression. Metabolic reprogramming of cancer cells is usually orchestrated by dysregulation of metabolic enzymes. It is generally thought that dysregulated metabolic enzymes contribute to cell cycle progression mainly by providing essential metabolites to meet the cellular needs for biosynthesis and bioenergenesis. However, it has been increasingly appreciated that metabolic enzymes can also conduct noncanonical or nonmetabolic functions that are referred to as “moonlighting” functions during certain physiological and pathological processes. Nonetheless, it is unclear if metabolic enzymes can directly control mitosis via metabolism-independent mechanisms.
On February 10, Prof. FENG Yuxiong and Prof. LU Zhimin from the Institute of Translational Medicine, Zhejiang University and Prof. LIANG Tingbo from the First Affiliated Hospital of the Zhejiang University School of Medicine co-published an article entitled “Glutamine synthetase licenses APC/C-mediated mitotic progression to drive cell growth” in the journal Nature Metabolism. This study illustrates how glutamine synthetase (GS) promotes cell mitosis in an APC/CCDC20-dependent manner and opens up a new avenue to understanding the crucial role of GS in cancer cells deeply and systematically.
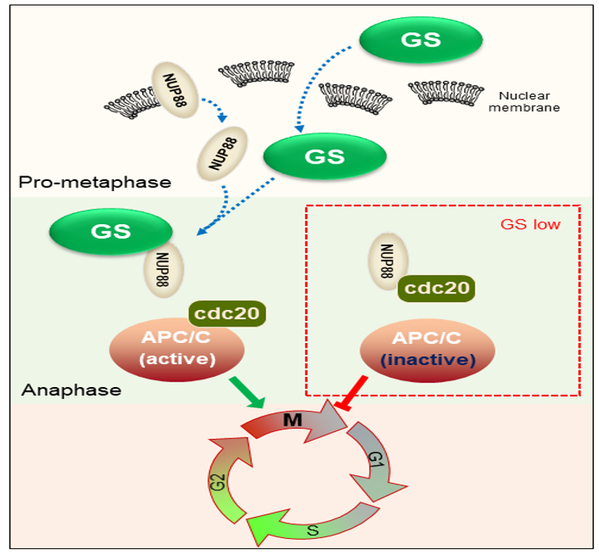
GS is essential for cancer cells to support their avid growth and survival. As the most abundant amino acid in plasma, glutamine is central to the metabolic network and serves as a critical nitrogen and carbon donor for the biosynthesis of essential metabolites. The researchers report that GS promotes cell proliferation by licensing mitotic progression independently of its metabolic function. Mechanistically, GS directly interacts with the nuclear pore protein NUP88 to prevent its binding to CDC20, thus activating the CDC20-mediated anaphase-promoting complex or cyclosome to ensure proper metaphase-to-anaphase transition. In addition, GS enhances the resistance of cancer cells to microtubule drugs independently of its catalytic activity.
“This study reveals the new function of GS in regulating the mitotic progression of tumor cells in a metabolic function-independent manner and provides a potential target for the treatment of tumors,” said Prof. Feng.