The microbiota-gut-brain axis in bipolar depression with multi-omics analysis
Bipolar disorder (BD) is a common, chronic, and devastating psychiatric disorder affecting 2-3% of the global population, characterized by recurrent episodes of depression and/or (hypo)mania, and accompanied by significant cognitive and social impairment, which brings a heavy disease burden to patients, families and society. However, the etiology of BD is complex, and the clinical diagnosis and treatment of BD faces great challenges.
In recent years, the relationship between gut microbes and human health and disease has attracted increasing attention. The bidirectional regulation mechanism between the brain and the gut, namely the microbiota-gut-brain(MGB) axis, has become a hot field of neuroscience research. Blood, as a transporter of metabolites, may act as a communication bridge between the brain and gut microbes. Growing evidence suggests a close relationship between BD and MGB axis dysfunction, but the exact mechanisms involved are unclear. The method of multi-omics combined analysis has opened up a new research model for in-depth understanding of the important role of the MGB axis in the pathophysiological process of BD.
On April 20, the team led by Prof. HU Shaohua from the First Affiliated Hospital, Zhejiang University School of Medicine and others published the latest research findings in Molecular Psychiatry. This study is the first to reveal the dysregulation of microbiota-gut-brain axis in the pathogenesis during a depressive episode of BD through combined analysis of blood metabolomics, fecal metagenomics, and brain network omics, suggesting that gut microbiota affects brain function by regulating blood metabolomics.
Based on the combined analysis of serum untargeted metabolomics, fecal metagenomic sequencing, and resting-state functional magnetic resonance imaging, a total of 109 patients with BD depressive episodes and 40 healthy volunteers were recruited in this study. Serum levels of metabolites, including short-chain fatty (SCFAs) derivatives, kynurenine (KYN), kynurenic acid (KYNA), gamma-aminobutyric acid (GABA), riboflavin, and folic acid, were decreased, while levels of spermine, gamma-glutamylcysteine, succinic acid, malic acid and indolepyruvic acid were elevated, which were mainly involved in tryptophan metabolism, arginine and proline metabolism, citric acid cycle, glutathione metabolism, fatty acid biosynthesis, pantothenic acid and coenzyme A biosynthesis, and SCFAs metabolism, suggesting that serum metabolic levels in BD patients changed significantly.
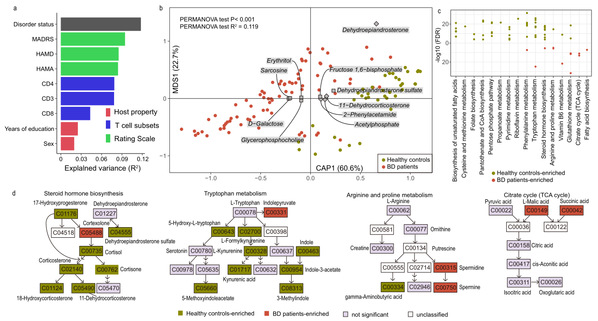
Fig. 1: Altered metabolites in serum of BD patients compared to healthy controls.
In the BD patient group, the alpha-diversity of gut microbes decreased, while the beta-diversity increased. Meanwhile, the microbial composition and functional characteristics were also changed. The relative abundance of Streptococcaceae and Bacteroidaceae was increased, while the abundance of Akkermansia muciniphila and Faecalibacterium prausnitzii was decreased. There were also significant inter-group differences in metabolic pathways involving amino acid metabolism and vitamin synthesis, suggesting that gut dysbiosis existed in patients with BD depressive episodes.
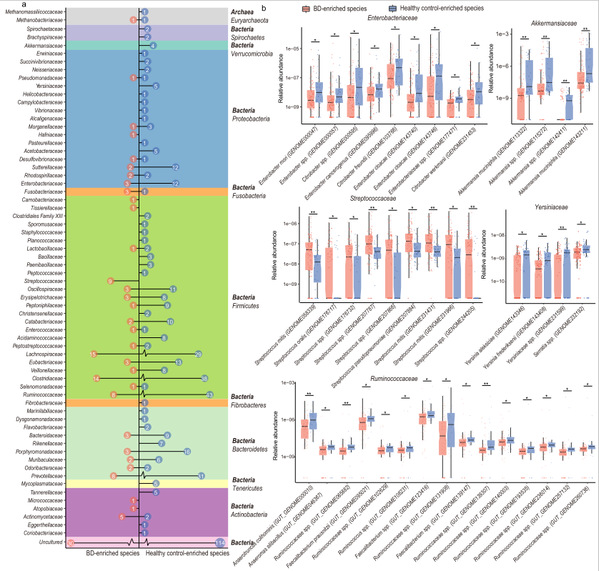
Fig. 2: Gut microbiota species associated with BD compared to controls.
In addition, further correlation analysis showed that gut microbes were significantly related to serum metabolites. For instance, Akkermansia muciniphila, Faecalibacterium prausnitzii, etc., were closely related to metabolic pathways of B vitamins such as folic acid, riboflavin, SCFAs, KYN, and GABA.
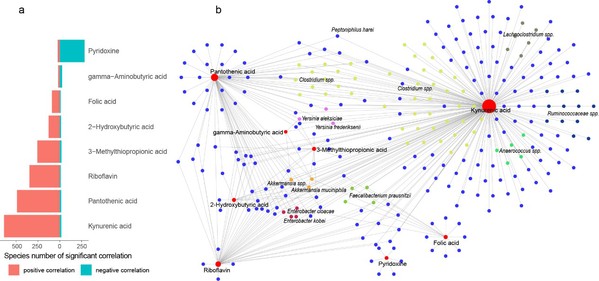
Fig. 3: The gut microbes significantly correlated with neuroactive metabolites.
Moreover, the combined analysis of multi-omics also found that the effect of serum metabolites on brain functional activity in BD patients was greater than that of gut microbes, suggesting that serum metabolites may mediate the regulatory effect of gut microbes on the brain. That is, gut microbes and their metabolites may transmit signals to the central nervous system through the circulatory system, thereby regulating the functional activities of the brain and participating in the pathogenesis of BD depression.
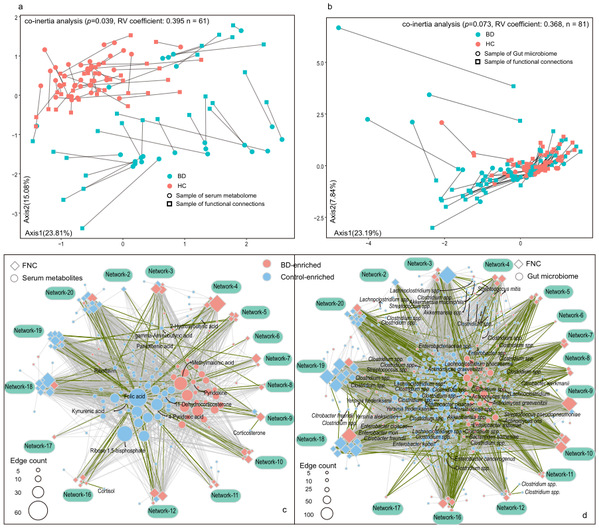
Fig. 4: Gut microbiota and serum metabolome of bipolar disorder correlate with brain functional connectivity.
In conclusion, the emergence of multi-omics integration research has overcome the limitations of a single omics, which is helpful for a deep understanding of the interaction between various molecules, mining effective biomarkers, and promoting the understanding of the complex etiological mechanism of diseases. The good application prospect is a new measure for in-depth research on the pathological mechanism of heterogeneous diseases, especially mental diseases in the future. This study was based on the method of multi-omics integrated analysis, explored the pathophysiological mechanism alongside the MGB axis of BD, and identified potential biomarkers for diagnosis and treatment of BD.
More information: Dr. LAI Jianbo and Dr. ZHANG Peifen from the First Affiliated Hospital, Zhejiang University School of Medicine, and Dr. LI Zhiming and Dr. DING Jiahong from Shenzhen BGI are the co-first authors. Prof. NIE Chao from BGI Shenzhen, Prof. SONG Xueqin from the First Affiliated Hospital of Zhengzhou University, Prof. Karsten Kristiansen from the University of Copenhagen and Prof. Susanne Brix from the Technical University of Denmark are the co-corresponding authors.
Source: The First Affiliated Hospital, Zhejiang University School of Medicine