Label-free super-resolution approach to imaging living cells
It is human nature to seek higher resolution to zoom in on a photo that interests us. However, this magnification is far from infinite, and our curiosity may be demotivated by a blurred pixel point that cannot be resolved more clearly. Unfortunately, the intrinsic property of light, i.e., optical diffraction, imposes this limitation.
Breaking the limit of optical diffraction is one of the most formidable challenges in the scientific community. A classic example of tackling this challenge is the development of super-resolution fluorescence microscopy, which received the 2014 Nobel Prize in Chemistry. Scientists have extended the border of optical microscopic imaging to the nanometer scale thanks to fluorescent molecules – often referred to as “labels.”
The team led by Prof. ZHANG Delong at the Zhejiang University School of Physics developed a label-free super-resolution approach—photothermal relaxation localization (PEARL) microscopy—on the strength of “extracting spatial features from the frequency domain.” PEARL microscopy can extract sub-diffraction-limit features directly from the location-dependent modulation of the probe beam in photothermal microscopy. As a result, it does not have to rely on fluorescent labels to break the limit of optical diffraction to reach 120 nm resolution.
The relevant findings were published in an article titled “Super-Resolution Imaging of Non-fluorescent Molecules by Photothermal Relaxation Localization Microscopy” in the journal Nature Photonics on January 23.
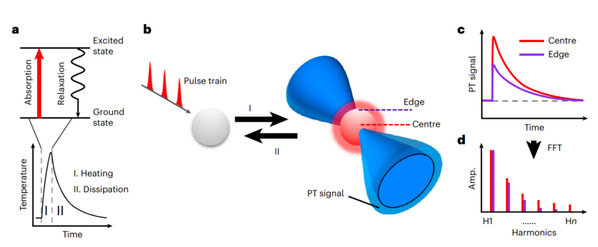
Fig. 1: The concept of PEARL imaging.
We can use the mirage phenomenon as an avenue to understanding PEARL. A mirage occurs due to refraction when light travels through air layers with different densities. “PEARL introduces a ‘lens’ into the target object so that the imaging system can capture the ‘deflection’ information of electromagnetic waves to obtain a precise image,” said Prof. Zhang.
When molecules encounter electromagnetic waves with specific wavelengths, they absorb electromagnetic energy and excite molecular vibrations. The same principle is utilized in a microwave oven to heat food. When electromagnetic waves vanish, the heated molecules enter an energy dissipation phase through heat dissipation and mechanical wave, known as photothermal relaxation. When it comes to the biological cells that scientists want to observe, the same type of molecules absorbs the same amount of energy, but their photothermal effect differs spatially. In general, the temperature rises more at the center and less at the edges. However, when electromagnetic waves disappear, the heat at the edges dissipates faster than that at the center.
“We use photothermal relaxation to establish links between the absorption features and spatial locations, and localize specific molecules by capturing high-frequency differences in photothermal relaxation processes,” Prof. Zhang introduced. “Let’s imagine the stars ‘twinkling’ in the night sky. It is actually light deflections caused by uneven atmospheric densities. PEARL technology can be likened to distinguishing the details of the stars via their ‘twinkling’ speed.”
The PEARL system consists of a pump beam and a probe beam. The pump beam is a pulsed, wavelength-tunable laser used to excite specific molecules, and it is focused on the target object, inducing molecular photothermal relaxation; the probe beam is raster scanned and perturbed by a sequence of ‘thermal lenses’ formed by photothermal relaxation. This perturbed signal contains location and spectra information. Due to the presence of the ‘thermal lens,’ the perturbed probe beam is temporally and spatially modulated, leading to a breakthrough in the extraction of finer spatial structures at higher harmonic frequency, which can break the diffraction limit of the probe beam.
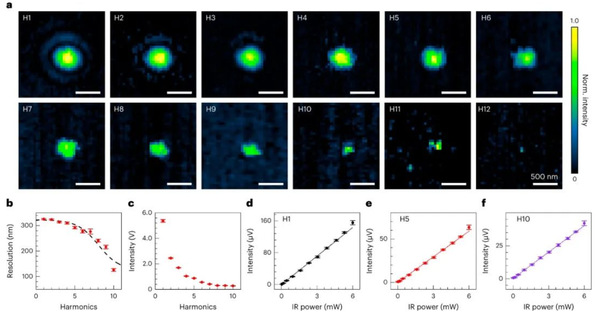
Fig. 2: Performance of V-PEARL imaging at higher harmonic demodulation.
ZHANG Delong et al. demonstrate how PEARL can be used to observe lipid droplets (LDs), an essential organelle in yeast cells. Yeast is a powerful model organism in biology. Individual yeast cells are about 2–4µm in size, in which the LDs are tiny, typically 0.05-0.5 µm, thus making traditional infrared imaging methods unable to visualize living cells in a high-resolution manner. The researchers adopt PEARL microscopy to image yeast cells with subcellular far-field infrared vibrational imaging. Their work not only demonstrates the distribution of intracellular LDs, but also determines their size distribution. In addition, PEARL reveals a fine spatial separation structure between small-sized LDs and a pair of protein droplets (about 170 nm each). “The smallest feature observed in the V-PEARL imaging of yeast is 86 nm. It is the first time that the sub-100-nm features have been resolved by far-field infrared imaging, said FU Pengcheng, co-author of the study.
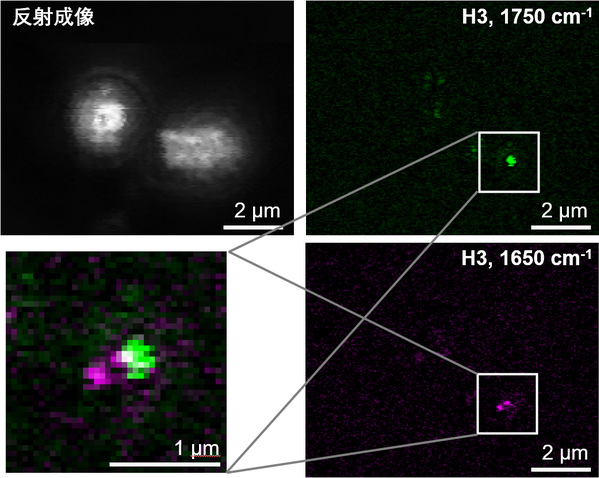
Fig. 3: V-PEARL imaging of live S. cerevisiae yeast cells.
In comparison with the widely-used fluorescence-based imaging methods, label-free imaging obviates the need for labeling and potential cytotoxicity. However, the existing non-fluorescent super-resolution imaging methods often rely on saturation effects or nonlinearity and are limited to a selection of molecules, which restricted their application. “The label-free super-resolution method we develop breaks these limitations, Prof. Zhang stressed. We believe that PEARL may open up new avenues for the super-resolution imaging of non-fluorescent molecules, promising exciting and broad applications in biology, medicine, and materials science.
Related report:
Super-resolution microscopy ditches fluorescent tags for gentler imaging of live cells
Image credit: The research team led by Prof. ZHANG Delong