ZJU scientists’ work opens door to potential T1D autoantigen-based vaccine
Type 1 diabetes (T1D) is an autoimmune disease stemming from the destruction of insulin-producing pancreatic β-islet cells. The incidence of T1D is on the rise worldwide, with about 5 out of every 100 diabetes patients having T1D. Among children and adolescents, this proportion hits a staggering 80%-90%. Major efforts have focused on insulin and associated neoantigens as a putative autoantigen, which may, however, trigger frustratingly elusive and complex results. Therefore, there is a compelling need for the development of new therapies for T1D patients.
As early as 2019, Prof. ZHOU Ruhong at the Zhejiang University College of Life Sciences and his colleagues discovered a new autoantigen—X-autoantigen, also termed as X-idiotype (Cell, 177, 1583-1599, 2019). This antigen was found to bind more favorably to HLA-DQ8 than insulin and its mimic (insulin superagonist) by about 1000-fold, but its molecular mechanism remains ambiguous.
On April 11, 2023, Prof. ZHOU Ruhong’s team published an open-access article titled “A mutagenesis study of autoantigen optimization for potential T1D vaccine design” in the journal PNAS. In this work, the researchers probed into the interaction of X-autoantigen and HLA-DQ8 and designed a series of enhanced-reactive pHLA-TCR antigens, thus opening up new vistas for more effective, safer and more customized therapy.
The researchers simulated the dynamic interaction between X-autoantigen and HLA-DQ8. They also designed (single) point, swap and double mutations in relevant epitopes to obtain new mutants that could enhance the binding affinity of antigens and HLA. Free energy perturbation (FEP) calculations revealed that the mutations of residue Tyr6 at antigen-binding site p6 play a critical role in improving the binding affinity of antigens for HLA. A moderate correlation between mutant affinity and residue volume, but no correlation with Eisenberg hydrophobicity. Further analyses indicated that there was a modest correlation between residue volume and mutant affinity, whereas Eisenberg hydrophobicity had no correlation with affinity.
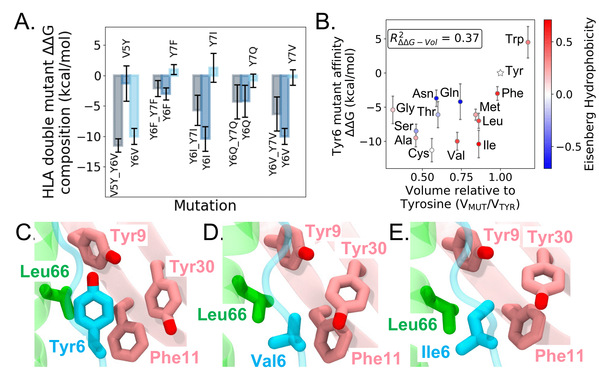
Fig. 1:X-autoantigen double mutation decomposition and Tyr6 mutation free energy results.
The researchers further explored the effect of antigen mutations on pHLA-TCR binding. FEP calculations showed that Tyr6 mutants Y6C and Y6I could enhance the binding of pHLA to TCR and could thus be the candidate for regulating TCR functions. Meanwhile, unfavorable TCR binding could reduce the risk of autoimmune diseases, indicating mutants V5Y_Y6V and Y6Q_Y7Q might work better. On the whole, mutations of p6 into smaller hydrophobic residues could improve the binding affinity of X-autoantigen to HLA-DQ8. Other p6 mutants (Y6V), the p5-p6 swap mutant (V5Y_Y6V) and the p6-p7 double mutant (Y6Q_Y7Q) could be used as potential therapeutic agents to modulate pHLA-TCR binding.
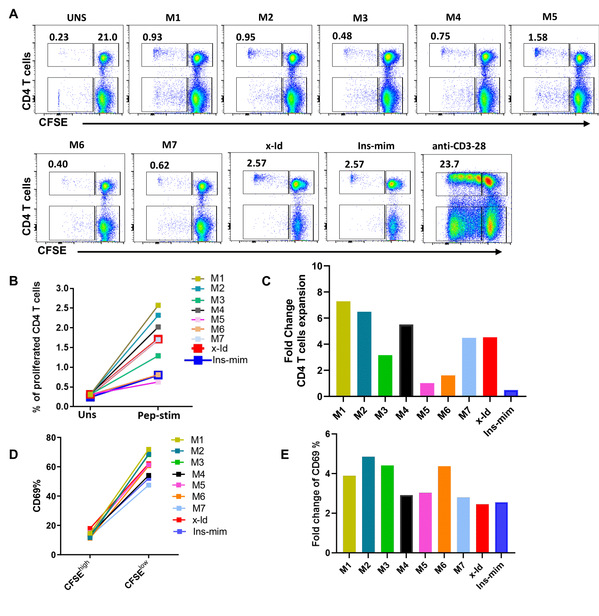
Fig. 2:Experimental CD4 T cell proliferation assays of X-autoantigen mutants
This work offers an insightful analysis of the mutation of X-autoantigen found in T1D patients and provides an optimized approach to designing novel antigens, thus opening up a new avenue for autoantigen-based immunotherapy.