How SARS-CoV-2 SL3 RNA element bind to human TIA1 protein?
The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) resulted in over 800 million infections and approximately 7 million deaths, posing a tremendous threat to mankind and radically altering the political, economic and public health landscape of the world. SARS-CoV-2 is a single-stranded plus RNA virus. Due to the RNA nature of its genome, the RNA virus would co-opt host RNA-binding proteins (RBPs) to perform its particular function during transcription and replication. For instance, it has been confirmed that several host RBPs participate in multiple events during infection, including affecting the recruitment of plus viral strands for replication, regulating the switch from translation to replication, and controlling viral RNA synthesis and stability. Therefore, a deep understanding of the interactions between viral RNAs and host RBPs will provide new insights into the virus life cycle and facilitate the development of novel antiviral strategies.
To this end, Prof. ZHOU Ruhong from the Zhejiang University College of Life Sciences and Prof. WANG Jianwei from the Chinese Academy of Medical Sciences & Peking Union Medical College conducted a collaborative study. Their findings were published in an article entitled “Mutagenesis and structural studies reveal the basis for the specific binding of SARS-CoV-2 SL3 RNA element with human TIA1 protein” in the journal Nature Communications on June 22.
Using RNA structure predictions and electrophoretic mobility shift assays (EMSA), the researchers identified the remarkable binding affinity between the SARS-CoV-2 SL3 RNA element and the human TIA1 protein. They also employed molecular models and MD simulations to construct a three-dimensional structure of SL3 and TIA1 protein, in which aromatic stacking, hydrogen bonds, and hydrophobic interactions combine to direct this specific binding.
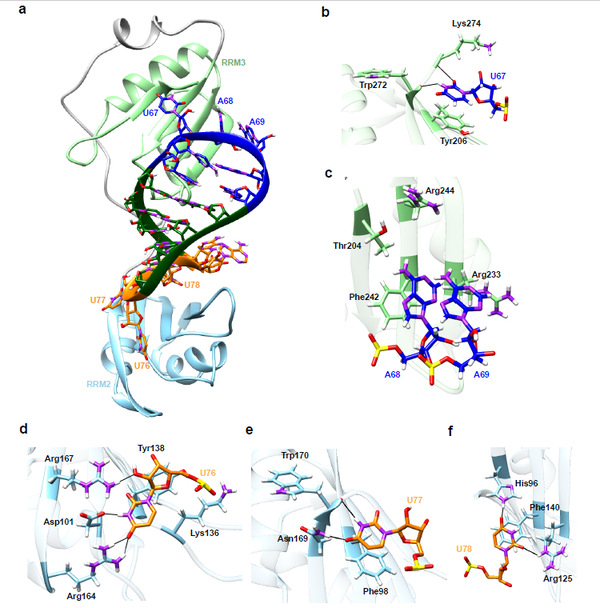
Fig. 1: The putative 3D binding model for SARS-CoV-2 SL3 RNA element and human TIA1 complex.
In addition, the researchers measured the binding affinities for various mutated and truncated variants of SL3 in order to determine the binding mode of the SL3 RNA element with the human TIA1 protein. They found that the 7-nt hairpin loop and the 5-nt 3’-terminal loop are indispensable for the binding. Both their length and their sequence have a marked impact on the binding affinity between SL3 and TIA1 protein. To further validate the functional impact of the SL3 element, the researchers designed several antisense oligonucleotides (ASOs) to perturb the interactions between SL3 and TIA1 protein. They observed a significant decrease in SARS-CoV-2 RNA yield in Huh7.5.1 cells (human liver cancer cell line).
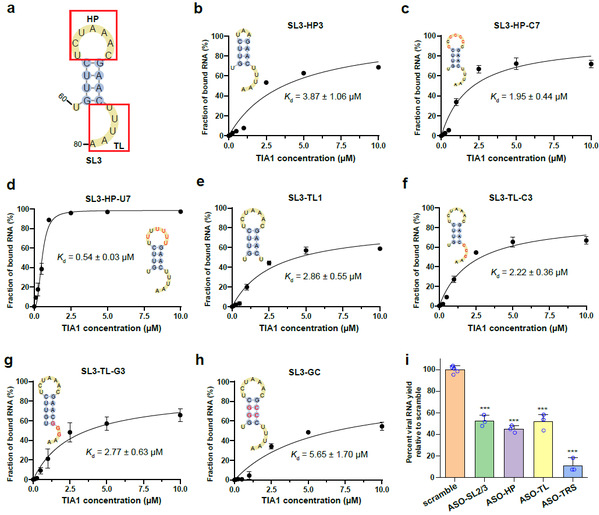
Fig. 2: Binding capabilities of various SL3 RNA variants with human TIA1 protein.
Moreover, given the fact that the 5’-UTR of the SARS-CoV-2 RNA genome is highly structured and harbors several stem-looop elements conserved among the betacoronavirus lineage, the researchers identified the interactions between the SL3 element in such viruses as the Middle East respiratory syndrome (MERS) coronavirus and the SARS coronavirus and the human TIA1 protein as well as their high binding affinity.
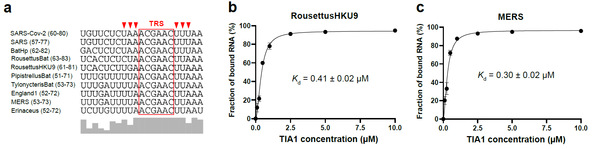
Fig. 3: Sequence comparison of SL3 RNA elements in betacoronavirus genomes and their binding abilities to human TIA1 protein.
This study opens up an avenue for exploring the viral RNA-host protein interactions and serves as a pioneering structural basis for RNA-targeting antiviral drug design.