Researchers developed weekly long-acting smart insulin
Researchers at Zhejiang University have developed an intelligent formulation of insulin that could achieve sustained blood sugar-responsive insulin release. After one subcutaneous injection, the formulation formed a pea-sized depot that can remain effective for over a week with negligible hypoglycemia risk.
The study, published in Nature Biomedical Engineering, was conducted by a research team led by GU Zhen, the Distinguished Chair Professor and Dean of College of Pharmaceutical Sciences at Zhejiang University. In the future, it may be feasible to have a single insulin injection last for one week or even longer, dynamically and effectively controlling blood sugar levels.

Study team (from right: Professor WANG Jinqiang, Professor GU Zhen, Ph.D. students ZHANG Juan and WANG Yanfang), photo: RAO Ping
Insulin, a hormone produced by islet beta cells in the pancreas, regulates blood sugar, helps store glycogen, and promotes fat synthesis. For the hundreds of millions of people with type 1 diabetes and part of type 2 diabetes, insulin has become an indispensable treatment to regulate blood sugar. However, they typically need to inject insulin subcutaneously multiple times per day to maintain stable basal and postprandial blood sugar levels, which undoubtedly imposes a huge burden on the daily life. In addition, due to the narrow therapeutic window of insulin, inappropriate insulin dosage may lead to hypoglycemia, posing a safety risk.
The beta cells can sense the concentration of glucose and regulate the secretion of insulin accordingly to adjust the glucose metabolism rate. By mimicking the function of beta cells, this formulation could achieve real-time sensing of blood sugar fluctuations and controlled release of insulin release. Under the normoglycemic condition, insulin release is slow and steady to maintain basal and normal blood sugar levels. When hyperglycemia occurs, glucose-responsive molecules could sense the increased blood sugar and trigger the rapid release of insulin, lowering blood sugar to a normal range.
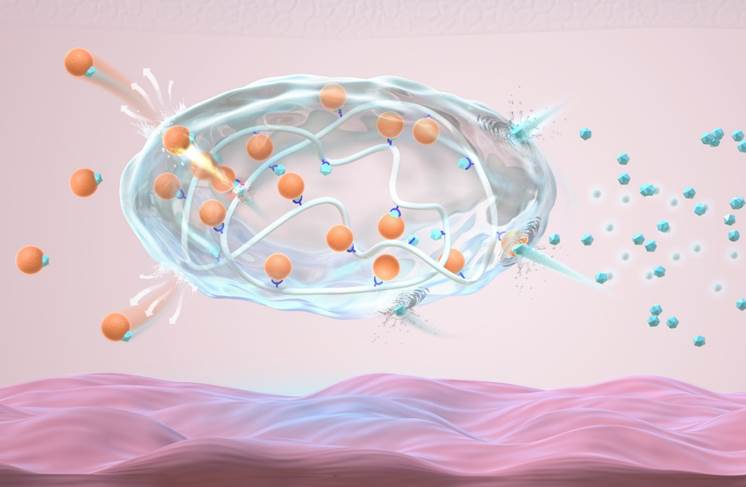
Schematic of the injectable glucose-responsive long-acting insulin formulation (orange dots: insulin; blue dots: glucose)
“The formulation is a white powdery complex consisting of polymers with glucose-responsive molecules (fluorobenzoborate-modified poly-ʟ-lysine) and gluconic acid-modified recombinant human insulin. Current study in the minipig model indicate that this new formulation has long-lasting and glucose-responsive insulin release properties. It could maintain normal blood sugar levels in 30 kg type 1 diabetic minipig model for over one week with a single dose, without symptoms of hypoglycemia,” said Gu. This complex forms an insulin “reservoir” when injected subcutaneously, which is beneficial for long-term storage of insulin under the skin and allows for rapid release when blood sugar levels rise.
“This is an exciting breakthrough in the development of next-generation insulin. In the past decades, many researchers are trying to make insulin usage convenient and safe,” said Prof. John Buse, the co-author of this work and a Professor and Director of the Diabetes Care Center at the University of North Carolina School of Medicine.
Injectable or implantable drug formulations or devices containing biomaterials often carry a risk of inducing the formation of fibrous capsules and affecting the function of the formulations or devices, such as inhibiting drug release. Of note, this complex forms a drug reservoir with the size of a pea in the subcutaneous adipose layer. As insulin is slowly released, the related polymer material is also absorbed.
“This is an interesting feature. Because of the unique properties of the materials and dosage form we have developed, the formation of a fibrous capsule can be inhibited,” explained by WANG Jinqiang, Ph.D., the co-corresponding author of this study and assistant professor of the College of Pharmaceutical Sciences at Zhejiang University.
The team plans to further assess the long-term biocompatibility of this formulation before bring it to clinical trials.
Source: College of Pharmaceutical Sciences, Zhejiang University