Teams from ZJU and MIT propose for the first time the concept of macroencapsulated bacteria
In a recent collaboration, Assistant Prof. NAN Kewang, Prof. GU Zhen from the College of Pharmaceutical Science at Zhejiang University (ZJU), along with Prof. Robert S. Langer from the Massachusetts Institute of Technology (MIT), published a review paper Macroencapsulated bacteria for in vivo sensing and therapeutics in the peer-reviewed journal Matter. The article is one of the first in the field that defines the concept of “macroencapsulated bacteria”. Co-first authors are LYU Yidan and SU Yuyan from College of Pharmaceutical Science, and HUANG Hao from College of Chemical Engineering at ZJU.
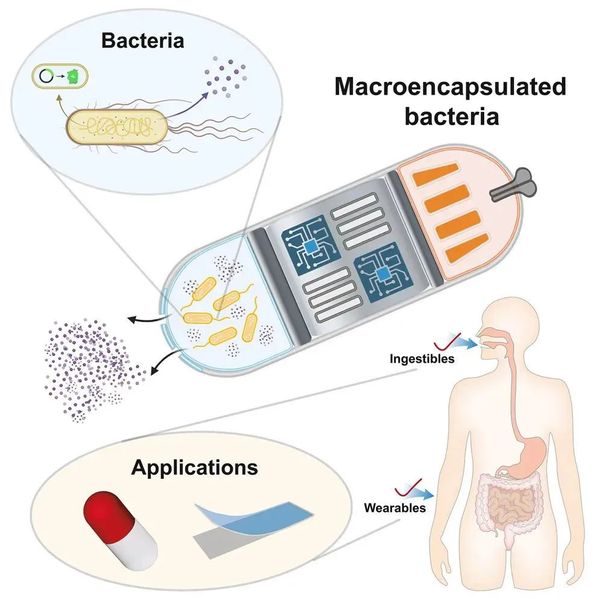
For years, it has been acknowledged that engineered bacteria possess unique capabilities, such as continuous drug production and the ability to penetrate deep tissues. These capabilities have the potential to significantly enhance local treatment concentrations, while mitigating the risk of systemic toxicity. Despite these advantages, their clinical application has been stymied by numerous challenges, including low in vivo viability rate, immune responses and ecological niche adaptation limitations.
This review introduces macroencapsulated bacteria as a potential solution, which involves encapsulating bacteria within millimeter to centimeter-scale systems to safeguard them from adverse internal conditions and avoiding immune responses. The microencapsulation of engineered bacteria potentially improves their localization and effectiveness at targeted sites and facilitate complete removal after the therapy.
Macro- v.s. Micro-encapsulations
The authors categorize existing bacterial encapsulation systems into two types: micro- and macro-encapsulation. The former is defined as surface modification of individual bacteria, focusing on methods to regulate gene expression and encapsulate single cells to enhance bacterial targeting and in vivo activity. Although more heavily studied, most microencapsulation methods are subject to limitations such as strain-specificity, bio-safety concerns including uncontrolled proliferation, and inability to actively process bio-signals from the sensory bacteria. In contrast, an emerging strategy combines millimeter to centimeter-scale engineered devices with bacteria (referred to as macroencapsulation). This method uses carriers such as hydrogel matrices, microneedles, capsules, and electronic devices to assist bacterial therapy via transdermal, oral, and other routes, creating the next generation of integrated bacterial devices. Compared to microencapsulation, macroencapsulation systems are platform-compatible with multiple strains, use approved materials and sizes, reducing the overall cost and burden of obtaining FDA approval. Additionally, their portability and integration with other functional units make them promising in wearable device development. The integration of wireless electronic components allows for remote control of bacteria and reception of sensing signals. Furthermore, macroencapsulation is beneficial for post-treatment bacterial clearance by physically isolating bacteria from spreading into tissues, thereby enhancing the safety of bacterial therapy.
Despite these advantages, research on macroencapsulation of bacteria is still in its infancy, with much fewer comparative literature with microencapsulation. Inspired by existing microencapsulation designs, the article outlines some feasible design standards, materials, and components for macroencapsulation. Although mainly conceptualized and in the early exploratory stage, macroencapsulation can provide more controllable and otherwise unavailable functionalities, such as isolation, residency, navigation, and communication (Figure 1).
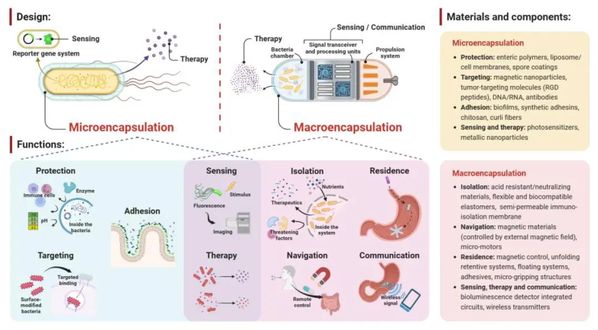
Figure 1. Design principles of bacterial encapsulation systems
Advancing therapeutic and diagnostic capabilities
Various forms of bacteria have demonstrated sensing and therapeutic effects through oral, subcutaneous, and intratumoral routes. Among these delivery methods, macroencapsulation bacteria are mainly applied via oral (edible) or epidermal (wearable) routes. Compared to microencapsulation bacteria administered intratumorally or intravenously, the application of macroencapsulation bacteria on tumors is much less. This may be because macroencapsulation bacteria lose their tumorigenicity (autonomous movement), which is crucial for the anti-tumor efficacy of bacterial therapy. However, recent studies have used macroencapsulation cells to treat tumors, which may imply the potential application of bacteria in tumor therapy (Figure 2).
Wearable systems: Due to their extremely low invasiveness, controllable removability, and accessibility for reading bioluminescent signals, the epidermal and transdermal routes have become a focus of research for bacterial sensing and therapy. In these applications, macroencapsulation provides portable carriers for protection, isolation, and enhanced sensing and therapeutic functions.
Transdermal systems: For transdermal drug delivery involving bacteria, the main forms of macroencapsulation include hydrogel patches and microneedles. Bacterial hydrogel patches are typically used to promote wound healing by carrying bacteria capable of secreting therapeutic substances. These patches may use thermoresponsive hydrogels to control the gelation time, allowing a low-viscosity gel at room temperature to undergo phase transition at body surface temperature, better adhering to the skin and immobilizing the bacteria inside. Additionally, photo-induced cross-linking has been studied to produce an in-situ hydrogel network at the wound site. Active hydrogel systems combining photodynamic antimicrobial properties are also under development.
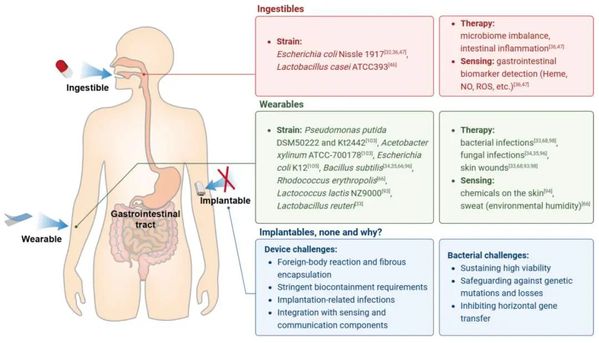
Figure 2. Macroencapsulated bacterial strains commonly used in different drug delivery pathways and their applications
Approaches to oral bacterial engraftment
Introducing bacteria into the human body is the first step, while achieving their sensing or therapeutic effects requires long-lasting and effective in vivo colonization. Successful bacterial engraftment in vivo is demonstrated through appropriate responses and secretion functions, depending on the bacteria's activity, colonization ability, and residency time. Although many engineered bacteria reported are administered orally, they are subjected to attacks from digestive enzymes, gastric acid, and the host immune system before reaching the small intestine (the most common implantation site of gastrointestinal microbiota), significantly reducing their viability and chances of successful implantation. To address these issues, various material-based methods have been developed to protect active substances and large molecules, including micro-motors, microcapsules, and surface coatings. However, upon reaching the expected location in the gastrointestinal tract, they still face multiple challenges, such as resistance from the host microbiota and the rapid turnover of intestinal epithelial cells, further hindering long-term implantation. Existing literature has employed methods such as multicellular encapsulation, single-cell modification, gene editing, native bacteria, and bacterial interactions to achieve longer bacterial colonization times (Figure 3).
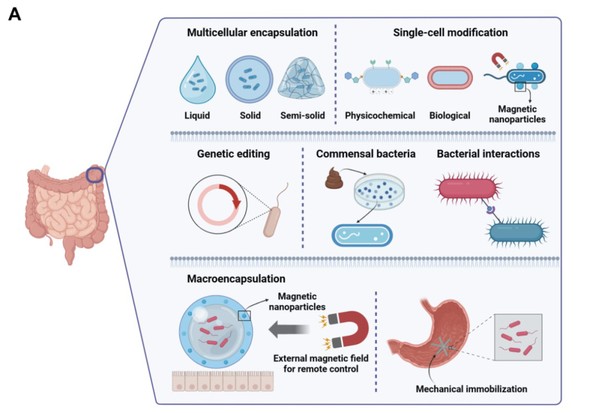
Figure 3. Different methods of oral bacterial coloniaztion
The Future
As the research on macroencapsulated bacteria is in its nascent stages, the study outlines feasible design standards, materials, and components while highlighting the necessity for interdisciplinary collaboration to overcome emerging challenges. The integration of electronic components for enhanced sensing and control functions, alongside the development of remotely responsive systems, paves the way for a new paradigm in medical treatment.
This collaborative effort between Zhejiang University and MIT represents a significant milestone in the journey towards realizing the full potential of bacterial therapies, offering a glimpse into a future where engineered bacteria play a central role in advancing healthcare.