ZJU Scientists Elaborates on Mechanism of LncRNA in Wiring up Hippo and Hedgehog Signaling to Reprogram Glucose Metabolism
Breast cancer has long been one of the major diseases for women. Every year, at least 400,000 patients die of this cancer all over the world. Cases in China account for 12.2% of all newly diagnosed breast cancers and 9.6% of all deaths from breast cancer worldwide.
The research team led by Prof. Lin Aifu of the College of Life Sciences, Zhejiang University, carries out relevant research in LncRNA. The Hippo pathway plays essential roles in organ size control and cancer prevention via restricting its downstream effector, Yes-associated protein (YAP). Previous studies have revealed an oncogenic function of YAP in reprogramming glucose metabolism, while the underlying mechanism remains to be fully clarified. Accumulating evidence suggests long noncoding RNAs (lncRNAs) as attractive therapeutic targets, given their roles in modulating various cancer-related signaling pathways. The research team discovers that lncRNA breast cancer anti-estrogen resistance 4 (BCAR4) is required for YAP-dependent glycolysis. Mechanistically, YAP promotes the expression of BCAR4, which subsequently coordinates the Hedgehog signaling to enhance the transcription of glycolysis activators HK2 and PFKFB3. Therapeutic delivery of locked nucleic acids (LNAs) targeting BCAR4 attenuated YAP-dependent glycolysis and tumor growth. The expression levels of BCAR4 and YAP are positively correlated in tissue samples from breast cancer patients, where high expression of both BCAR4 and YAP is associated with poor patient survival outcome.
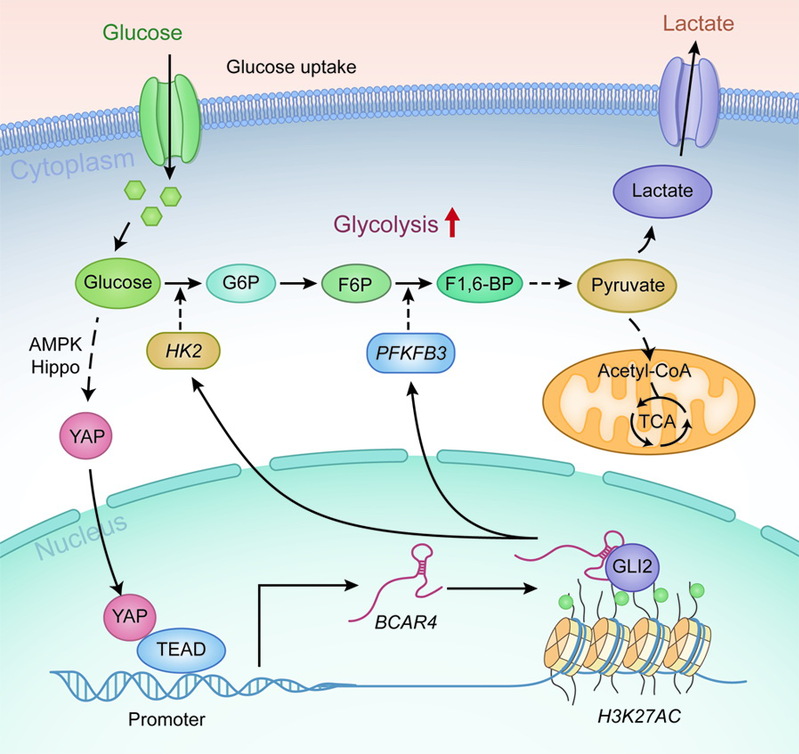
This study not only reveals the mechanism by which YAP reprograms glucose metabolism, but also highlights the therapeutic potential of targeting YAP-BCAR4-glycolysis axis for breast cancer treatment.
Their research findings are published in the EMBO Journal.





