For IVF treatment, choose what's right!
In the field of assisted reproductive technology (ART), preimplantation genetic testing for aneuploidy (PGT-A) is increasingly employed as an adjunct to morphological assessment with the aim of selecting chromosomally normal embryos, thereby potentially improving treatment outcomes. However, it remains unclear whether PGT-A improves live birth in couples with severe male factor infertility undergoing intracytoplasmic sperm injection (ICSI). A recent study published online by The BMJ on December 23, 2025 conducted across four reproductive centers in China, led by Professor HUANG Hefeng, has provided crucial evidence to inform this clinical question.

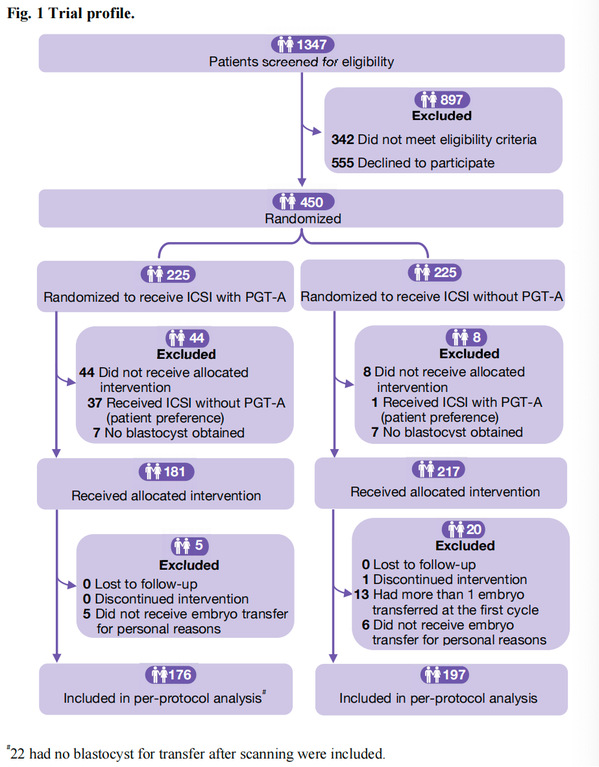
Between 15 July 2018 and 6 January 2023, the trial enrolled 450 couples with severe male factor infertility. Participants were randomly allocated to either ICSI with PGT-A (n=225) or ICSI alone without genetic testing (n=225). The primary outcomes were live birth after the first embryo transfer and cumulative live birth rate resulting from up to three transfer cycles within 12 months after randomization. Primary analysis was based on the intention-to-treat principle. In total, 109 couples in the PGT-A group (48.4%) and 104 couples in the no PGT-A group (46.2%) had a live birth after the first embryo transfer (odds ratio 1.09 (95% confidence interval (CI) 0.76 to 1.58), P=0.64). The cumulative live birth rates per woman were 60.4% (136/225) and 60.9% (137/225) in the PGT-A and no PGT-A groups, respectively (0.98 (0.67 to 1.43), P=0.92). The PGT-A group had significantly lower rates of pregnancy loss after the first embryo transfer (13 (5.8%) PGT-A group v 43 (19.1%) no PGT-A group, 0.26 (0.14 to 0.50), P<0.001) and cumulative pregnancy loss (25 (11.1%) v 51 (22.7%), 0.43 (0.25 to 0.72), P=0.001) than the no PGT-A group.
These results demonstrate that the chromosomal selection enabled by PGT-A does not translate into a higher rate of live-born infants. However, significantly lower rates in pregnancy loss in the PGT-A group were observed, both after the first transfer (5.8% vs. 19.1%) and cumulatively (11.1% vs. 22.7%). This reduction was driven primarily by decreases in biochemical and first-trimester clinical pregnancy losses. This finding aligned with the mechanistic rationale for PGT-A by identifying and avoiding the transfer of aneuploid embryos.
Despite this reduction in pregnancy loss, the lack of improvement in live birth rates suggests a complex interplay of factors. The results of trophectoderm biopsy could not completely represent the genetic composition of the inner cell mass of the blastocyst, which is the precursor to the embryo. Whether an embryo is able to achieve a live birth not only depends on an assumed normal chromosome constitution, but also on other factors such as gene expression, cellular mechanisms, laboratory conditions, etc. Trophectoderm biopsy was conducted only in women who underwent PGT-A; to date, there is no sound evidence from prospective randomized controlled trials regarding the impact of embryo biopsy on maternal and neonatal health. PGT-A could reduce the chance of a live birth, but this harm is balanced by an increased chance of an euploid embryo being transferred. The lower birthweight of newborns from singleton pregnancies in the PGT-A group in our study also suggested that potential adverse effects of PGT-A did not necessarily lead to pregnancy loss, but did likely lead to a less optimal pregnancy outcome. Additionally, medical costs are an essential factor that needs to be considered during the decision making process when discussing treatment strategies.

Therefore, we suggested that the need for PGT-A should be carefully considered for patients with severe male factor infertility. Further studies are needed to evaluate more precise indications for PGT-A in assisted reproductive technology practices.
Source: School of Mdeicine, Zhejiang University
Editor: HAN Xiao





